BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://journal.zums.ac.ir/article-1-6052-en.html


 , Mehdi Rajabi1
, Mehdi Rajabi1 

 , Alireza Moraveji2
, Alireza Moraveji2 

 , Ehsan Shafiei Rad1
, Ehsan Shafiei Rad1 

 , Mehrdad Mahdian *3
, Mehrdad Mahdian *3 


2- Dept. of Community Medicine, Kashan University of Medical Sciences, Kashan, Iran
3- Trauma Research Center, Kashan University of Medical Sciences, Kashan, Iran ,
✅ The results showed that prophylactic administration of dexamethasone and ketamine is effective in relieving shoulder pain after a CS.
Caesarean section (CS) is the most common abdominal surgery performed on women worldwide (1, 2). This type of surgery is associated with several complications ranging from extended hospital stays to maternal death (3, 4). One of the most common complications after a cesarean delivery is shoulder pain, which is often not treated. The incidence rate of this complication ranged from 22.6% to 46.3% in different studies (5-7). This pain has a sharp, deep, and referential nature and usually resolves within 2-3 days after surgery (8). This pain may be caused by sub-diaphragmatic clotting, sub-diaphragmatic air trapping, or peritoneal irritation arising from the two previous reasons (9).
Shoulder pain is observed not only after a CS but also after other abdominal and chest surgeries (8, 10, 11); thus, it can be considered a common complication, especially after laparoscopic surgery (12-14). Shoulder pain in some patients can be more excruciating than the pain of surgical incision (15). This pain can be associated with diaphragmatic irritation/injury and phrenic nerve by local acidosis, the irritative impact of carbon dioxide during pneumoperitoneum, or stretching forces on the diaphragm (15, 16). Although carbon dioxide is not used in CS compared to laparoscopic surgeries, the stretching forces on the diaphragm during CS can be one of the causes of shoulder pain in such patients (9). However, far too little attention has been paid to CS-related shoulder pain.
In one study, Zirak N et al. (2012) evaluated the role of anesthetic technique in the incidence of shoulder pain after CS. According to the results of their study, mothers who received general anesthesia were significantly more likely to develop shoulder pain than those in the spinal anesthesia group (7). Abbas et al. (2017) conducted a randomized clinical trial and concluded that intravenous ketorolac just before the surgery could significantly reduce the intensity and incidence of intraoperative shoulder pain in patients with CS (9).
Research to date has tended to focus more on shoulder pain relief after laparoscopic abdominal surgeries rather than after a CS. Tsai H et al. (2011) reported that the incidence of shoulder pain after laparoscopic surgery decreased significantly following saline injection into the peritoneal cavity (17). Asgari Z. et al. (2012) also reported a significant decrease in the intensity of shoulder pain in patients who received intraperitoneal dexamethasone after gynecologic laparoscopic surgeries (18). In contrast to these studies, Sutchritpongsa P. et al. (2013) showed that the intraperitoneal instillation of bupivacaine plus morphine does not decrease postoperative shoulder pain incidence after gynecologic endoscopy (19).
Ketamine is a well-known anesthetic drug, and its clinical usefulness has expanded into the management of a wide range of conditions, including acute and chronic pains (20). Dexamethasone is the most commonly prescribed corticosteroid for pain (21). Considering the increasing prevalence of CS under spinal anesthesia and the high prevalence of shoulder pain in patients with CS, this study was conducted to examine the effect of intravenous dexamethasone and ketamine on reducing shoulder pain in these patients. As far as the researchers investigated, there is no published study to assess the impact of intravenous ketamine and dexamethasone on shoulder pain after CS.
Study Design
This cohort study was conducted among 231 subjects in 2016. The Research Ethics Committee of Kashan University of Medical Sciences, Iran approved the study (code: 6007; date: March 4, 2015; research grant number: 93234). All subjects were referred to Shahid Beheshti University Hospital in Kashan, Iran.
Sampling Method and Sample Size Calculation
A convenience method of sampling was used in the study. Subjects were eligible if they had a full-term singleton pregnancy, met the criteria for he American Society of Anesthesiologists (ASA) class I or II, and were nullipara. The following exclusion criteria were applied: multiparity, a history of previous laparotomy, sensitivity to the drugs used in the study, shoulder pain before CS, receiving analgesic drugs before CS, underlying diseases such as renal or circulatory diseases, and any severe complications that occurred during the surgery (e.g., excessive bleeding, hysterectomy). Our sample size calculation (77 patients in each group) was based on a similar study that used dexamethasone for alleviating shoulder pain resulting from gynecologic laparoscopy and a type I and II error of 0.05 and 0.20, respectively (18).
Patient Recruitments and Study Protocol
Based on different approaches used by anesthesiologists to treat shoulder pain after CS, participants (n=77) were divided into three groups as follows: dexamethasone recipients, ketamine recipients, and control (no medication).
The present study was designed to investigate the possible effect of ketamine and dexamethasone in the prevention of CS-related shoulder pain in patients who underwent spinal anesthesia. Before the anesthesia, patients were given 500 ml of a solution of lactated ringer. Spinal anesthesia was carried out using a 25-gauge needle after skin prep using 10% povidone iodine prep solution. Hyperbaric bupivacaine 0.5% (15 mg) was used as an anesthetic solution, which was administered between L4-L5 or L5-S1. All participants received oxygen at a rate of 5 L/min during the procedure.
After spinal anesthesia and before surgical prep and drape, patients received preventive medications based on the anesthesiologists’ preferred approach. In the first group, patients were treated with 0.5 mg/kg intravenous ketamine; in the second group, patients were treated with 0.1 mg/kg dexamethasone intravenously. The third group of patients (control) did not receive any medication.
A checklist including each patient’s demographic characteristics and possible shoulder pain during CS, immediately after CS, and 1 h, 6 h, 12 h, 18 h, and 24 h post-operation was completed using a visual analogue scale (VAS). Each VAS was a 10-cm horizontal line graph; the words “no pain” and “worst pain” were on the furthermost left and at the right ends of the graph, respectively. As such, the most severe pain was denoted by a score of 10 cm (15). Patients and the resident responsible for filling the checklist were unaware of the grouping.
In the presence of moderate and severe pain, patients received 25 mg pethidine intravenously. Total pethidine consumption in the 24-h postoperative period was recorded.
Statistical Analysis
A one-way analysis of variance (ANOVA) was used to compare demographic data. Chi-square tests were used for categorical variables to assess the differences between the two groups of participants. Statistical analysis of the post-intervention results was performed using a repeated-measures ANOVA that compared VAS changes over time. Differences in analgesic doses in the experimental groups were also compared using ANOVA. Results were reported as means (± SD). P values of ≤0.05 were considered significant.
Although 231 patients were initially considered in the study, five patients (three from the control group and two from the Ketamine group) lost to follow-up (The patients who drop out). No significant difference was seen between the three groups regarding their demographic and hemodynamic data (Table 1). The repeated-measures ANOVA showed the impact of time on pain scores (P<0.001).
Table 1. Demographics and hemodynamic data of the study participants
| P-value* | Control | Ketamine | Dexamethasone | Variables |
| 0.414 | 25±4.3 | 26.4±4.2 | 27.1±4.4 | Age |
| 0.716 | 38±0.9 | 38±1.0 | 38.1±1.0 | Gestational age (year) |
| 0.616 | 75.8±11.7 | 74.7±8.7 | 76.4±11.3 | Weight (Kg) |
| 0.261 | 120.7±11.1 | 123.1±9.7 | 122.2±10.8 | SBP (mm Hg) |
| 0.271 | 76.8±9.5 | 77.5±8.8 | 77.4±8.6 | DBP (mm Hg) |
| 0.496 | 97.2±15.3 | 97.8±13.4 | 100.3±15.2 | PR (bpm) |
*ANOVA
Abbreviations: SBP; systolic blood pressure (at admission), DBP; diastolic blood pressure (at admission), PR; pulse rate (at admission), bpm; beat per minute
The time-group interaction was also significant regarding the pain score variations indicating inter-group differences in VAS changes over time (P<0.003) (Table 2 and Figure 1).
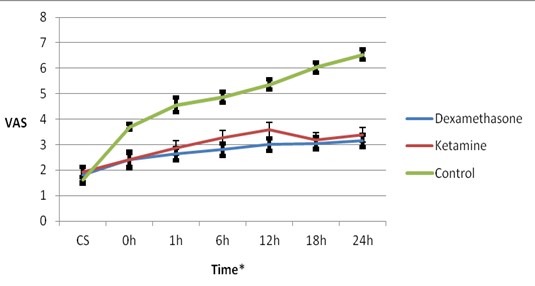
Figure 1. Mean and 95% confidence interval of visual analogue scale (VAS) at different times in study groups.
*Time: CS; during cesarean section, 0; immediately after and,1, 6, 12, 18 and 24 hours after cesarean section
Table 2. Visual analogue scale of shoulder pain among study groups over the time
| VAS | Groups | P-value1 | P-value2 | |||
| Dexamethasone | Ketamine | Control | Time effect | Time*group effect | ||
| CS | 1.83±1.47 | 1.89±1.12 | 1.62±0.47 | 0.306 | 0.001 |
0.003 |
| 0 | 2.43±1.47 | 2.36±0.93 | 3.70±0.50 | 0.157 | ||
| 1 | 2.65±1.11 | 2.86±1.28 | 4.56±1.28 | <0.001 | ||
| 6 | 2.83±1.11 | 3.26±1.28 | 4.86±0.92 | <0.001 | ||
| 12 | 3.04±1.12 | 3.59±1.28 | 5.36±0.92 | <0.001 | ||
| 18 | 3.07±1.11 | 3.16±1.28 | 6.03±0.92 | <0.001 | ||
| 24 | 3.17±1.11 | 3.38±1.28 | 6.54±0.90 | <0.001 | ||
-
One Way ANOVA 2. Repeated Measures ANOVA
Abbreviations: VAS, Visual Analogue Scale; VAS CS, VAS during cesarean section; VAS 0, VAS immediately after cesarean section; VAS 1, 6, 12, 18, and 24, VAS after 1, 6, 12, 18 and 24 hours after cesarean section
At the beginning of the study (i.e., during CS), the incidence of pain in the dexamethasone group was 13%; this rate decreased to 2.6% after 24 h. Comparable incidence changes were also observed in the ketamine group such that the incidence of pain decreased from 13.3% at the beginning of the procedure to 4% after 24 h of operation. However, in the control group, the incidence of pain started at 8.3% and increased to 33.3% after 24 h (Table 3).
Regarding post-cesarean shoulder pain, the least significant difference (LSD) post-hoc tests showed a significant difference between the dexamethasone and ketamine groups and between the dexamethasone and control groups (P<0.05). No significant difference was found between the dexamethasone and ketamine groups regarding reductions in shoulder pain (P>0.05).
The number of pethidine recipients among the control group was significantly higher than in the two other groups (P<0.001). There was no statistically significant difference in the amount of 24-h pethidine used to control shoulder pain in all three groups (P=0.635).
Table 3. Frequency of pain distribution in study groups at different times
| P-value1 | Groups | Time(hours) | ||
| Control | Ketamine | Dexamethasone | ||
| 0.57 | 6 (8.3) | 10 (13.3) | 10 (13) | CS |
| 0.72 | 12 (16.7) | 13 (17.3) | 10 (13) | 0 |
| 0.07 | 15 (20.8) | 10 (13.3) | 6 (7.8) | 1 |
| 0.012 | 13 (18.1) | 6 (9) | 3 (3.9) | 6 |
| <0.001 | 24 (33.3) | 12 (16) | 3 (3.4) | 12 |
| <0.001 | 27 (37.5) | 5 (6.7) | 4 (5.2) | 18 |
| <0.001 | 24 (33.3) | 3 (4) | 2 (2.6) | 24 |
Table 4. Pethidine usage and cumulative pethidine (in mg) consumed in study groups
| Pethidine Intake2 | Pethidine Intake1 (mg) Mean±SD |
Groups | |
| No | Yes | ||
| 66 (85.7) | 11 (14.3) | 29.4±9.8 | Dexamethasone |
| 63 (84) | 12 (16) | 29.5±10.1 | Ketamine |
| 44 (61.1) | 28 (38.9) | 31.25±11.3 | Control |
| <0.001 | 0.635 | P-value | |
-
ANOVA Test
-
Chi-square Test
Discussion
The findings of this study revealed that the prophylactic administration of dexamethasone and ketamine was effective in the relief of shoulder pain associated with CS. The effects of these two drugs were comparable and were more effective than administering no medication. Also, pain intensity (VAS) during the study period varied from the time of cesarean delivery until 12 h after CS. No significant difference was detected between the dexamethasone and ketamine groups after 12 h.
In clinical research on CS complications, shoulder pain is usually overlooked. To the best of our knowledge, this subject has not been addressed well in the literature, especially in interventional studies. A randomized, prospective, double-blinded study conducted by Abbas et al. (2017) investigated the impact of ketorolac on decreasing the severity and prevalence of intraoperative shoulder pain in CS patients. They concluded that intravenous administration of 30 mg ketorolac before surgery could significantly reduce the severity and incidence of intraoperative shoulder pain in patients with CS while also significantly reducing requests for analgesics for severe pain in the shoulder tip in the ketorolac group in comparison to the control group (1).
Moreover, some studies have investigated the prevalence or incidence of such pain. Cift et al. investigated shoulder tip pain (STP) in patients with CS and evaluated the incidence between spinal and general anesthesia. They found that the overall incidence of STP in the study population (300 patients) was 35.7%. The incidence of STP in the spinal anesthesia group (26.6%) was significantly lower than in the general anesthesia group (43.9%) (6). In a similar study, Zirak et al. (2012) pointed out that shoulder pain was very common in patients undergoing CS, with a prevalence rate of 39.45%. Similar to what was mentioned in the above study, shoulder pain was more prevalent in patients who received general anesthesia than in those who received spinal anesthesia (7). In another study, and in patients with CS who received combined spinal-epidural anesthesia, Kikuchi et al. (2014) found that women undergoing CS under combined spinal-epidural anesthesia experience STP with great frequency (46.3%) (5). These findings, along with our results, support the idea that shoulder pain due to CS is common.
The mechanism of shoulder pain during CS has not been properly explained yet. Shoulder pain is also reported after laparoscopic surgeries [5, 6]. Peritoneal irritation and stretching have been identified as possible causes of this pain. In addition to visceral manipulation and peritoneal mopping, retained blood clots in patients with CS can cause diaphragmatic irritation and stimulate the phrenic nerve. This might explain the shoulder pain experienced by these patients (1).
Several studies have been conducted to reduce shoulder pain after laparoscopic surgeries. Tsai et al. (2011) reported a significant decrease in shoulder pain after laparoscopic surgery following saline injection into the peritoneal cavity (17). In a similar study, Asgari et al. reported reduced shoulder pain severity resulting from the peritoneal cavity administration of dexamethasone after gynecologic laparoscopy (18).
Our findings are consistent with those of Asgari et al. The only difference is that they administered dexamethasone in the peritoneal cavity instead of intravenously in their study. However, the findings of the current study do not support the study of Sutchritpongsa P. et al., in which they reported that the sub-diaphragmatic administration of bupivacaine and morphine did not affect the relief of shoulder pain in gynecologic laparoscopy (19). These differences can be explained in part by methodological differences, the nature of surgery, and, to some extent, the type of medication used.
Limitations
There were two significant limitations in this study. First, assessment of patients was performed within only the first 24 h after operation because most patients were discharged on the second day after their operation. The patients should be followed up after their discharge from the hospital to determine how long shoulder pain lasts after surgery. Second, the impact of shoulder pain was not considered during postoperative recovery. Therefore, the current study does not indicate the extent to which shoulder pain limits patients’ performance after CS. Further studies, preferably clinical trials, are needed to answer this question.
Conclusion
According to the findings of the present study, the prophylactic administration of dexamethasone and ketamine may be effective in relieving shoulder pain after CS. The effects of each of these drugs were comparable, and they were significantly more effective than administering no medication.
Acknowledgements
The authors are grateful to the Deputy of Research of Kashan University of Medical Sciences for its financial support in this study (Grant No: 93234).
Ethical considerations
This study was approved by the ethics committee of Kashan University of Medical Sciences (Ethical letter No. 6007, March 4, 2015). All procedures conducted in studies with human participants were according to the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Funding and support
Tthis study has been supported by the Deputy of Research of Kashan University of Medical Sciences, Kashan, Iran (Grant No: 93234).
Conflicts of Interest
Authors declared no conflict of interests.
Received: 2020/06/8 | Accepted: 2020/10/16 | Published: 2020/11/14
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |



