BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://journal.zums.ac.ir/article-1-6141-en.html


 , Fahimeh Mohammadghasemi2
, Fahimeh Mohammadghasemi2 

 , Fahimeh Shams *3
, Fahimeh Shams *3 

 , Sina Saliminasab1
, Sina Saliminasab1 

 , Paridokht Karimian4
, Paridokht Karimian4 

 , Morteza Rahbar Taramsari5
, Morteza Rahbar Taramsari5 

 , Siroos Kordrostami6
, Siroos Kordrostami6 

 , Ali Alavi Foumani7
, Ali Alavi Foumani7 

 , Hossein Hemmati8
, Hossein Hemmati8 

 , Seyed Amineh Hojati9
, Seyed Amineh Hojati9 

 , Pirouz Samidoust10
, Pirouz Samidoust10 

 , Masoumeh Faghani2
, Masoumeh Faghani2 


2- Cellular & Molecular Research Center, Guilan University of Medical Sciences, Rasht, Iran
3- Student Research Committee, School of Medicine, Guilan University of Medical Sciences, Rasht, Iran ,
4- Dept. of Pathology, School of Medicine, Guilan University of Medical Sciences, Rasht, Iran
5- Dept. of Forensic Medicine, School of Medicine, Guilan University of Medical Sciences. Rasht, Iran
6- Razi Hospital, School of Medicine, Guilan University of Medical Sciences, Rasht, Iran
7- Inflammatory Lung Diseases Research Center, Dept. of Internal Medicine, Razi Hospital, School of Medicine, Guilan University of Medical Sciences, Rasht, Iran
8- Dept. of General Surgery, Division of Vascular Surgery, Guilan University of Medical Sciences, Rasht, Iran
9- Gastrointestinal and Liver Diseases Research Center (GLDRC), Razi Hospital, Guilan University of Medical Sciences, Rasht, Iran
10- Dept .of General Surgery, School of Medicine, Guilan University of Medical Sciences, Rasht, Iran
✅ This study aimed to report histopathological features and serological outputs of the lung, heart and liver in a patient suffered from Coronavirus disease-2019 (COVID-19).
The disseminated Coronavirus disease-2019 (COVID-19) has almost affected the whole world in the recent months (1). The disease symptoms are variable from mild to severe. It was reported that different body organs are involved by infection, especially lungs and heart (2). It is known that the elderly and people suffering from chronic diseases are more vulnerable to COVID-19 infection (3). There is high similarity in the clinical manifestations of COVID-19 and severe acute respiratory syndrome Coronavirus 2 )SARS-CoV-2(; both enter into the tissues through a special receptor called angiotensin-converting enzyme 2 (ACE-2). This receptor is expressed mainly in the respiratory tract and also in various tissues such as kidney, gastrointestinal tract, liver, testis and endothelial cells (4). Therefore, it attacks to numerous tissues. In 23% of the patients, COVID-19 was associated with the cardiac injury (5). Abnormal liver function (57.8%) is also observed in the patients (6). In this case report, we described a COVID-19 patient, who died in the hospital after eight days of admission.
Case Report
The case study was a 64 year-old woman, who developed left limb bradykinesia in 2005, and was subsequently diagnosed with Parkinson's disease (PD). Later, she had bilateral tremor, rigidity and pain in both upper and lower limbs (left>right). She felt pain; complaining of rising from bed and turning back. The following medications were prescribed: Norstar (Levodopa-B) 250 mg BD, Tranqopine 25 mg BD, Clomipramine 10 mg nightly and Clonazepam 2 mg nightly. She had a background of smoking but quit more than five years ago. She had no history of respiratory, cardiovascular, renal and gastrointestinal diseases and alcohol consumption.
On the date of April 6th, 2020, she had some symptoms such as cough, dyspnea, fever and myalgia. Her PD symptoms worsened after fever. Concurrent occurrence of COVID-19 and fever, the patient had mild bilateral ptosis (left >right) that worsened later. General and motors' symptoms became more severe.
She referred to Razi Hospital. The COVID-19 real time PCR (RT–qPCR) test was performed for the nasopharyngeal specimen. The vital signs were reported as follows; respiratory rate (RR)=28/min, heart rate=130/min, temperature=39°C and blood pressure (BP)=140/100 mmHg. She was transferred to the intensive care unit (ICU). Noninvasive ventilation (with mask 10 Lit/min) was applied due to hypoxia and tachypnea.
She seemed ill and pale. The examination findings were as follows; RR=38/min, HR=136/min, BT=39/5 C, BP=110/60 mmHg. The arterial blood gas (ABG) analysis showed the following results; pH=7.26, PCO2=59 mmHg, PO2=55.6 mmHg, HCO3=26.2 mEq/L, O2 sat=83.3%, Be=-1.9 mmol/L and BB=46 mmol/L.
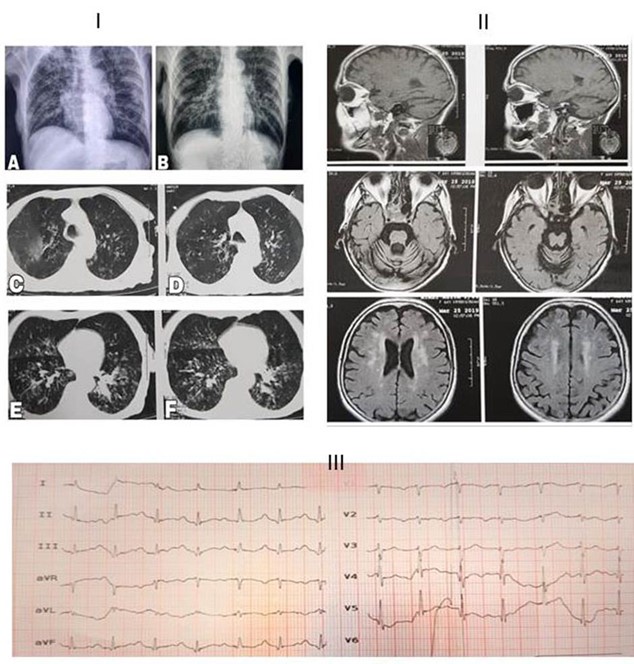
Some biochemical laboratory tests, chest X-ray, computed tomography (CT) and brain MRI were requested by physician (Table 1). Lung CT scan revealed bilateral multiple ground glass centrilobular nodules, predominantly in the posterior segment of upper lobes, upper segments of lower lobes and right middle lobe. Mild bronchial wall thickening and dilatation were also noted. No evidence of peripheral ground glass was observed in favor of typical COVID-19. Regarding the patient history and atypical COVID-19, aspiration pneumonia was considered as differential diagnosis.
The heart size was normal (Figure 1). The COVID-19 was suspected according to the chest X-ray. Therefore, Lopinavir, Ritonavir (Kaletra), Vancomycin and Meropenem were prescribed for the patient.
For controlling the PD signs, Levodopa was prescribed. The ECG results showed mild mitral valve and tricuspid valve regurgitation with an ejection fraction (EF) ratio of 55-60% (other issues were not notable). The cardiac enzymes were not checked, because there was no suspicion of cardiac involvement in the patient.
Table 1. Laboratory results of the patient during hospitalization
| Variable | Day1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 | Day 8 | Normal range |
|---|---|---|---|---|---|---|---|---|---|
| WBCs, hpf (U/A) | 19 | N/A | N/A | N/A | 26.4 | 29.9 | N/A | 33.4 | 4-11 |
| RBCs, hpf (U/A) | 4.02 | N/A | 3.34 | N/A | 3.17 | 4.04 | N/A | 3.89 | 4.5-5.1 |
| Hemoglobin,gr/dL | 9.4 | 9.6 | 7.9 | 8.7 | 6.4 | 10.3 | 8 | 6.3 | 12.3-15.3 |
| HCT | 31 | 29.9 | 25.5 | 27.2 | N/A | N/A | N/A | N/A | 35.9-44.6 |
| PLT | 372 | 362 | 377 | N/A | 263 | 169 | 212 | 204 | 150-450 |
| CRP, mg/L | +1 | +1 | N/A | N/A | N/A | +2 | N/A | N/A | Negative:<6 |
| Creatinine, mg/dL | 0.6 | 0.6 | 0.6 | 0.6 | 0.6 | 0.6 | 0.75 | 0.97 | 0.6-1.2 |
| BUN, mg/dL | 20 | 20 | 19 | 17 | 16 | 16 | 24 | 30 | 13-43 |
| Na, mEq/L | 134 | 137 | 140 | 143 | 140 | 138 | 172 | 141 | 135-145 |
| K, mEq/L | 4.1 | 3.8 | 3.9 | 3.6 | 3.5 | N/A | 4.2 | 4.9 | 3.5-5.3 |
| Mg, mEq/L | 1.7 | 1.7 | 2.2 | 3.8 | 2.1 | 1.7 | N/A | 2.3 | 1.53-2.5 |
| Ca, mEq/L | 9.2 | N/A | N/A | N/A | 9.2 | N/A | 9.7 | N/A | 8.6-10.3 |
| ALT (SGPT). U/L | 17 | 15 | N/A | 16 | 12 | N/A | 15 | N/A | <40 |
| AST (SGOT), U/L | 12 | 7 | N/A | 20 | 17 | 7.6 | N/A | N/A | <34 |
| ALP | N/A | 318 | N/A | 276 | 285 | 275 | N/A | N/A | 64-306 |
| LDH, U/L | 697 | 549 | N/A | N/A | N/A | 987 | N/A | N/A | 230-480 |
| Blood Sugar (BS) | 119 | 139 | 168 | 137 | 170 | 150 | 152 | N/A | <200 |
| Albumin, g/L | 2.8 | 2.2 | N/A | 2.4 | N/A | 3.1 | N/A | N/A | 3.5-5.5 |
| PT, seconds | 12.5 | 12 | 12 | 12.5 | 12 | 12 | 12 | 12 | 11-13.5 sec |
| PTT, seconds | 37 | 18 | 34 | 35 | 65 | 39 | 64 | 60 | 24-40 sec |
CRP: C-reactive protein; AST: Aspartate aminotransferase; ALT: Alanine aminotransferase, ALP: Alkaline phosphatase; LDH: Lactate dehydrogenase, PT: Prothrombin time; PTT: Partial thromboplastin time
Figure 1. I: Chest X-ray. A: before admission. B: during the admission time which showed faint patchy opacities in the right side and bilateral diffuse hyperinflation. C, D, E and F: The chest axial CT obtained by the time of admission; it revealed bilateral multiple ground-glass centrilobular-nodules in the lungs predominantly in posterior segment of the upper lobes, upper segments of the lower lobes and the right middle lobe. Mild bronchial wall thickening and dilatation were also noted. No evidence of peripheral ground glass in favor of typical COVID-19 was observed. II: Brain MRI. There was multiple small abnormal signal lesions in the brain white matter of periventricular space and sub-cortical of both hemispheres and also basal ganglia; cited alterations were due to small vessel ischemic disease and encephalopathy. Also, senile dilatation of CSF spaces and senile atrophy were seen. III: Electrocardiogram (ECG) showed occasional premature atrial contractions (PAC) and prolonged QT-interval accompanied with first-degree atrioventricular block.
On day 6, the patient consciousness reduced. Vital signs and ABG test assessment were as below: RR=22, pH=7.33, PCO2=49.3 mmHg, PO2=45.7 mmHg, HCO3=26 mEq/L, and O2 sat=78.3% (under mask ventilation of 10 Lit/min), Be=-0.4 mmol/L and BB=47.5 mmol/L. Finally, she was intubated and underwent mechanical ventilation (mode A/C, FIO2: 80%, rate 18/min, VT: 480 ml, and PEEP: 10 cmH2).At midnight on the last day, BP decreased to 91/49 mmHg; therefore, one-liter normal saline 0.9% was injected. BP reduced again, then 10 micro/min norepinephrine was injected, however, BP did not elevate (BP: 7.1/39 mmHg). Additionally, the heart rhythm changed from normal sinus rhythm (NSR) to atrial fibrillation (AF). The patient experienced severe hypotension, and acute respiratory distress syndrome (ARDS) developed. The O2 saturation did not reach higher than 70% (BP: 65/30 mmHg). She did not respond to the cardiopulmonary resuscitation. Finally, she passed away with septic shock diagnosis. A signed consent was obtained from the patient's family for the biopsy.
Histopathology
Tissue sampling was done from lung, heart and kidney by a non-automatic coaxial biopsy needle (16*20 G). The tissues specimens were sent to the pathology lab for histopathologic evaluations. The tissue samples were kept in 10% formalin solution and embedded by paraffin and stained by Haematoxylin-Eosin (H&E). The prepared slides were observed via a light microscope. Other parts of the tissues were sent to the virology lab for the COVID-19 detection by RT-qPCR.
Real-time PCR Assay for SARS-COV-2 in Tissues
Liferiver RNA isolation kit (Belgium) was used for the total RNA extraction. Based on the manufacturer's protocol, total RNA was extracted and RT-qPCR assay was conducted (Catalog number: 8.0131901X024E, Version.B1.0, China). Open reading frame 1ab, Nucleocapsid protein N and E genes were amplified via RT-qPCR.
Histopathology Results
Lung tissue needle biopsy showed increased thickness of the alveolar wall, fibroblastic ball and mild fibrosis in the interstitial tissue. Moreover, pneumocytes type II hyperplasia with giant cell formation in the lung alveolar wall was associated with inflammatory cells in the alveolar spaces; probably, mentioned alterations were due to compensatory response to produce more surfactant. These changes were nonspecific and corresponding to the organizing phase of pneumonia (Figure 2.I).
Histopathological observations of the heart tissue revealed mild inflammatory cell infiltration in both endomysium and perimysium with edema; this finding was consistent with mild myocarditis (Figure 2.II).
Wide sinusoids and congestion were observed in the parenchyma of the liver tissue. Micro-vesicular steatosis in periportal hepatocytes and hepatocyte size heterogeneity were observed. There was also mononuclear infiltration in the portal area. These findings were considered as nonspecific changes (Figure 2.III).
Rt-PCR Results
The test result was negative in terms of virus presence in the lung, liver and heart tissues.
Figure 2. I: Biopsy specimen of the lung tissue. The arrow shows a giant cell. *: Pneumocyte hyperplasia of type 2 with giant cell formation. II: autopsy specimen of the heart tissue. E: endomysium, C: cardiomyocyte, P: perimysium, *: inflammatory cells. III: Biopsy specimen of the liver tissue. Purple arrows: steatosis in hepatocytes. Black arrows: sinusoids, V: portal vein, B: bile duct. The blue rectangle shows inflammation around the portal area. Hematoxylin and eosin 400X.
Discussion
Both ACE2 and Dipeptidyl peptidase-4 are expressed in the central nervous system (CNS). Therefore, the risk of COVID-19 may be elevated in neurological damages such as Alzheimer's and Parkinson's disease. PD is the most common neurodegenerative disorder after Alzheimer and mostly affects elderly people (>60 years). In PD, the dopaminergic neurons are progressively loosed in the Substantia Nigra, both structurally and functionally; it leads to dopamine level reduction in the brain (14). The loss of movement control in PD leads to increased psychological stress gradually. On the other hand, stressful life puts people more susceptible to COVID 19 and respiratory distress; it is too early to decide whether COVID-19 can worsen the disease. This patient had PD for several years. Generally, premature death is higher in PD compared to the normal people. Some determinant factors such as pneumonia, cerebrovascular and cardiovascular diseases affect death occurrence in PD (15). Possibly, in this patient, the COVID-19 had altered the route of dopamine production in CNS, and therefore, it worsened the PD motor symptoms. However, we did not evaluate the patient for PD symptoms specifically (14). The RT-qPCR results were negative in all tissues. The clinical and pathological signs of COVID-19 patients in lung, heart and liver are unknown exactly, and further researches are needed.
Conclusion
The pathological biopsy showed advanced alveolar damage in the lung, peri-portal inflammation in the liver, spread steatosis in the hepatocytes and moderate myocarditis in the heart. However, the RT-qPCR test was negative for the obtained specimens of lung, liver and heart tissues. The alterations in the above-mentioned tissues may be due to the secondary side effects of the COVID-19 or pharmacological treatments.
Acknowledgements
We would like to express our thanks toward the medical staff of Razi Hospital, especially the general operating room, acute emergency and intensive care unit.
Funding and support
The informed consent was signed by the patient’s family; all procedures were conducted under the guidelines of Guilan University of Medical Sciences (Ethic code number: IR.GUMS.REC.1399.012).
Conflicts of Interest
We declare no competing interests.
Received: 2020/07/26 | Accepted: 2020/12/5 | Published: 2020/12/30
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |