BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://journal.zums.ac.ir/article-1-6320-en.html
2- Dept. of Physiology, Zanjan Branch, Islamic Azad University, Zanjan, Iran ,
✅ Exercising (treadmill running) and flaxseed oil prevent lead-induced behavioral deficits.
Environmental pollution from heavy metals, like lead, is one of the outcomes of industrial life. Long-term exposure to lead causes the emergence of oxidative stress in the brain, changes in the cell membrane, disruption in the signaling pathways, and damages in neurotransm-ission and synaptic activity (1, 2). Lead weakens learning and memory (3) and develops anxiety and depression (4). Given the wide range of lead use in industry, it seems that the sole way to deal with its harmful effects is to find ways that minimize damage.
Today, it is believed that a healthy body equals a healthy brain e. 30-60 minutes of exercise three days a week for six consecutive weeks causes a decrease in brain damage in patients suffering from brain injury (5). Treadmill running reduces oxidative stress in the brain (6). It also increases the level of neurotrophic factors, decreases expression of inflammatory factors and increases the level of anti-inflammatory factors, inhibits apoptosis (7), improves memory, and decreases anxiety and depression in rats suffering from posttraumatic stress disorder (8). Therefore, we hypothesized that treadmill running could probably reduce behavioral disorders resulted from lead acetate in rats.
An alternative measure to reduce brain damages is to use medicinal plants. These herbs consist of a significant amount of anti-oxidants, are affordable and easily available. Therefore, there is a wide range of studies on the subject of medicinal plants and their compounds. Flax (Linum usitatissimum L.) is an annual edible plant, the oil of which has abundant amounts of omega-6 and omega-3. Flaxseed oil contains a huge amount of α-linolenic acid, oleic acid, and α- and γ-tocopherol, etc. (9). Flaxseed oil has anti-inflammatory effects (10), reduces oxidative stress, inhibits cytotoxicity (11), increases monoamines level, and additionally decreases acetylcholine esterase activity in rats that have received lead acetate (12). Thus, it was hypothesized that flaxseed oil can probably reduce behavioral disorders resulted from lead acetate in rats.
Accordingly, the purpose of our research is to examine the effect of flaxseed oil and treadmill running on memory impairment, anxiety, and depression induced by lead acetate in male rats.
Sixty male rats (each 200-220 g) were kept in cages in groups of four. Animals had easy access to water and food, and were kept at 24 ᵒC, and 12h/12h light / dark period.
The rats were divided into 6 groups (all groups included10rats)as follows:
1- The Control (C, intact)
2-The Exercise (Ex)
3-The Flaxseed oil (FO)
4-The Lead (L)
5-The Lead- Exercise (L-EX)
6-The Lead- Flaxseed oil (L-FO)
Groups L, L-EX, and L-FO received 100 mg/kg lead acetate (Sigma, USA), and groups 3 and 6 received 4 ml/kg flaxseed oil (Adonis Gol Darou, Tehran, Iran) for 28 consecutive days (4, 13). Both lead acetate and flaxseed oil were administrated by oral gavage every day.
Before the start of the 4-week training period, groups 2 and 5 ran for 10 minutes daily at 5 km/h for 5 consecutive days to get acquainted with the treadmill (Tajhiz Gostare Omid Iranian, Tehran, Iran). For the next four weeks, they ran for 30 minutes every day for 5 days a week; in the first two weeks, 5 minutes at 13 m/min, 20 minutes at 16.5 m/min, and the last 5 minutes at 13 m/min. In the second two weeks, they ran for 5 minutes at a speed of 13 m/min; 20 minutes at a speed of 20 m/min, and then 5 minutes at a speed of 13 m/min (14).
The Morris water maze (MWM) test was performed on days 24-28 to investigate learning and spatial memory. The elevated plus maze (EPM) test and forced swimming test (FST) also were used to study the level of anxiety and depression at the end of the period.
The water maze consisted of a tank (130 cm cylinder and 60 cm high) placed in a half-dark room and filled with water. An invisible platform was placed beneath the 2cm of the water surface in the northeast of the tank. Multiple signs were placed nearby the tank. On days 24-27, rats swam in the tank 4 times a day for 90 s to find the platform. After finding the platform or at the end of the 90 seconds, each rat sat on the platform for 20 s until the start of the next round. The animals’ movement was recorded by the camera on top of the tank and data was transferred to MazeRouter software (Tabriz, Iran) to be analyzed. Reduction in time elapsed (s) and distance moved (cm) to reach the platform meant an enhancement of learning. On day 28, each rat swam in the tank (without a platform) for 60 seconds. An increase in time elapsed (s) in the target quarter (the location of platform in training days) meant memory improvement (15).
At the end of the period, the anxiety behavior was examined by EPM test. EPM contains four crossed arms (two open and two closed arms) at a distance of 50 cm from the ground. In this test, each rat was put in the central square of the arms, and its behavior was assayed for 5 minutes. The number of entries and duration of staying in the open arm were recorded by the camera on top of the system. An increase in the percentage of open-arm entries (OAE) and time spent in the open arms (OAT) showed anxiety reduction in the animal (4).
The depression behavior was studied by forced swimming test. Rats were put into a cylinder (40 × 18 cm i.d) including water at 24- 26°C. Each rat was placed in the column for 6 minutes. The first 2 minutes were for acquainting with the system and the behavior of the animal was analyzed in the next 4 minutes. An increase in immobilization time (s) meant an increase in the level of depression (4).
All the behavioral tests were carried out at 9-12 O’clock.
Results were presented as means ± SEM. One-way ANOVA was used for detection of the differences between groups. Factors related to the training of each group in the first to fourth days in the MWM test were compared by repeated measures. The variance among groups was defined utilizing the HSD and Tukey post-hoc tests. The p-value lower than 0.05 was significant.
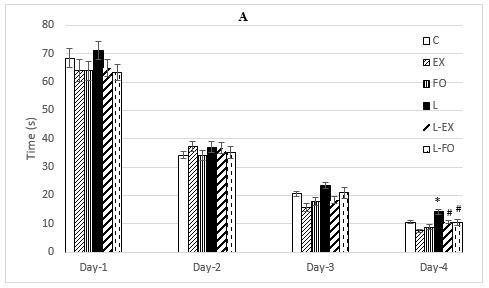
Kidney function was evaluated by measuring the kid- During the MWM test, the time and distance traveled to reach the platform, had a significant reduction on day 4 in comparison with day 1 (P < 0.001) (data not shown). 28days of lead acetate consumption caused learning reduction in animals so that they recorded more time and distance to reach the platform on day four compared with the controls (P < 0.05, P < 0.001 respectively). Treadmill running resulted in learning improvement in lead acetate received rats. These rats spent less time and distance to reach the platform compared with the Lead group (P < 0.05, P < 0.001 respectively). Flaxseed oil consumption also caused learning improvement in lead acetate received rats in a way that they spent less time and distance to reach the hidden platform compared with the Lead group (P < 0.05, P < 0.01 respectively). (Figure 1. A, B). After 4 days of training, the probe test was performed in one step (without a hidden platform). The probe test also showed memory impairment in the Lead group and its improvement in groups L-EX and L-FO in comparison with the L group, since compared to the Control group, they swam less time in this region (P < 0.05) and groups L-Ex and L-FO spent more time in the target quarter (P < 0.05) (Figure 1. C). The EX and FO groups did not differ significantly from the control group in any of the factors related to training and probe test (Figure 1).
Results of the EPM test showed anxiety in lead acetate received rats. The percentage of OAT and OAE in this group reduced compared with the controls (P < 0.05). Treadmill running and flaxseed oil consumption, both caused anxious behavior decline in lead acetate received rats so that the percentage of OAT in both groups had a meaningful increase compared with the L group (P < 0.01, P < 0.001 respectively) and a notable increase in OAE percentage (P < 0.05). Also, the OAT percentage decree-sed in EX and FO groups compared to the control group (P < 0.01 and P < 0.001, respectively) (Table 1). The percentage of OAE in these groups has no meaningful difference from the Control. Animals’ locomotor active-ties (sum of entrances to the open and closed arms) did not have a major difference in various groups (data not shown).
Figure 1. Time (A) and distance moved (B) to reach the platform in MWM test in 4 consecutive days. Time spent in target quarter in probe test (C). Results are shown as mean ± SEM. (N=10). * P <0.05 and *** P < 0.001 vs. group C and # P < 0.05, ## P < 0.01, ### P < 0.001 vs. group L.
According to FST, the 28- day treatment of lead acetate caused the development of depression in rats so that immobilization time in group L increased in comparison with the Control (P < 0.01). Treadmill running caused significant immobilization time reduction in lead acetate received rats (P < 0.05). Although the immobility time was shorter in the L-FO group compared to the L group, there was no significant difference (P > 0.05). Furthermore, no meaningful difference was found among EX and FO groups with the Control in this factor (Figure 2).
Table 1. Results of anxiety examination utilizing the elevated plus maze (EPM) Test.
| Group | OAT % | OAE % |
| C | 33.03 ± 2.47 | 46.10 ± 1.99 |
| EX | 46.23 ± 1.38 ** | 43.85 ± 1.10 |
| FO | 56.13 ± 1.50 *** | 49.18 ± 1.28 |
| L | 21.86 ± 3.54 * | 35.48 ± 3.62 * |
| L- EX | 35.16 ± 2.36 ## | 44.35 ± 1.73 # |
| L- FO | 45.86 ± 1.12 ### | 44.91 ± 2.10 # |
The percentage of the open arm time (OAT) and open arm entries (OAE) were determined. Results are shown as means ± SEM. (N=10). * P < 0.05, ** P < 0.01 and *** P < 0.001 vs. group C; # P < 0.05, ## P < 0.01 and ### P < 0.001 vs. group L.

Figure 2. Immobility time in forced swimming test (FST). Results are shown as means ± SEM (N=10). ** P < 0.01 vs. group C and # P < 0.05 vs. group L.
Discussion
The results showed that treadmill running causes improvement in spatial learning and memory in lead acetate-induced rats. Running plan with a treadmill like what was used in our study decreased the hypoxia hypobaric-induced damage in the CA1. Treadmill run-ning also reduced microglia and astrocytes activity in the hippocampus and inhibited nitric oxide synthase activity. Also, it increases the brain-derived neurotro-phic factor (BDNF) expression and, as a whole, increased the anti-oxidant and anti-apoptotic capacity of the hippocampus (14). Treadmill running excites neurogenesis in the dentate gyrus of old rats. It inhibits apoptosis in the hippocampus, improves rats perfor-mance in the MWM test (16), and also increases long-term potentiation in streptozotocin-induced diabetic rats (17). Therefore, all of these may perform a function in improving the memory of the L-Ex group.
We observed that spatial learning and memory in the L-FO animals improved compared with group L. Flaxseed oil decreases the activity of choline esterase in rats’ brains that received lead acetate (12). It also inhibits lipid peroxidation, oxidative stress, and histopathologic damages due to lead acetate (11). The positive effects of flaxseed oil on the memory of lead acetate -received rats may be due to rich stocks of omega3 and omega6; since alpha-linoleic (which can be found in flaxseed oil abundantly) increases neuro-genesis especially in the dentate gyrus in the hippocampus (18). On the other hand, feeding the rats on linoleic acid-enriched butter for 4 consecutive weeks results in passive avoidance memory impro-vement (19). Vitamin E not only strengthens the brain anti-oxidant system of hypothyroid rats but also increases BDNF level in their brain and improves the spatial memory of these animals (20). Additionally, omega 3 prevents lead-caused memory impairment in rats (21).
Results of the EPM test showed anxiety reduction in rats of group L-EX even the percentage of OAT in the EX group increased compared with controls and is in line with the result of studies by Mazur et al. (22). Activation of serotonergic neurons in the lateral raphe nucleus leads to anti-anxiety behavior. Elevation of corticotrophin-releasing hormone causes activation of the hypothalamus-pituitary-adrenal axis and then induces anxiety-like behaviors. It has been proved that mild exercise increases c-Fos expression in raphe sero-tonergic neurons and leads to anti-stress effects (23). Running increases the level of GABA neurotransmitter in the brain (24), so the anxiolytic effects of treadmill running in L-EX can be justified.
Just like exercising, flaxseed oil reduced anxious behavior in rats that received lead acetate. Even the percentage of OAT in group FO was higher than controls. Therefore, flaxseed oil has anxiolytic effects. These results are in line with studies carried out by Shallie et al. (25) based on the anxiolytic effects of flaxseed oil in rotenone-received rats. Additionally, there is a reverse relation between omega 3 (which can be found abundantly in flaxseed oil) and the emergence of anxiety (26). Furthermore, alpha-linoleic acid stimu-lates the GABAergic system in the basolateral nucleus of the amygdala and prevents anxious behavior which is resulted from brain injury (27). Therefore, the anxiolytic effect of flaxseed oil may be mediated by stimulating GABA transmission in the brain.
The results of FST also show that treadmill running causes depression reduction in lead acetate- received rats which is in line with the results reported by Patki et al., (8) on the anti-depression effects of treadmill running in rats exposed to stress. One of the major reasons for depression is apoptosis and neurodeg-eneration in the hippocampus or even its volume reduction (28), and treadmill running protects the hippocampus against neurodegeneration and apoptosis (11). Therefore, the anti-depression effect of treadmill running may be because it inhibits neurodegeneration. Treadmill running prevents dopamine level decline in the brain (29) and activates raphe nucleus serotonergic neurons (23). Given that lead reduces dopamine and serotonin levels in different parts of the brain (30), and reduction of these neurotransmitters in the brain is one of the most important reasons for depression (31), therefore, treadmill running prevents their reduction and consequently the appearance of depression. One of the reasons for changes in the level of neurotran-smitters in the depressed brain is the reduction of BDNF level (32) and since treadmill running increases BDNF expression and strengthens its signaling paths in the hippocampus (14), there is the probability that it prevents lead-induced BDNF level reduction in rats because exposing to lead decreases BDNF level in the brain (33).
Flaxseed oil additionally improved the performance of lead acetate- received rats in FST, but it didnot have significant effects. Flaxseed oil, in higher doses than what had been used in this study, could prevent the reduction of mono-amines levels in rats exposed to lead (12). Moreover, flaxseed oil prevents depression emergence in the first two weeks after birth, and this outcome is similar to the fluoxetine anti-depression effect (34) that can be related to the unsaturated fatty acid omega 3 in flaxseed oil. Omega 3 has an important role in curing depression (35). We suggest that higher doses of flaxseed oil or a longer period of consumption may prevent depression resulted from lead.
Conclusion
Finally, we concluded that treadmill running and flaxseed oil consumption reduce lead acetate behavioral deficits, and probably are good candidates to prevent and treat neuro damages resulted from lead.
Data availability
Raw data for this article is available upon demand.
Compliance with ethical guidelines
This study was approved by the ethics committee in Islamic Azad University- Zanjan Branch (approval number: IR.IAU.Z.REC.1396,31).
Statement of Ethics
All the rats were handled based on the Principles of Laboratory Animal Care (NIH publication No. 85-23, revised in 1985), and the study was approved by the Ethics Committee of Urmia University of Medical Sciences, Urmia, Iran.
Acknowledgements
Authors wish to thank Mr. Yaghoub Bigdeli for his help in performing behavioral tests.
Conflicts of Interest
All authors declare no conflict of interest.
Funding Sources
None.
Received: 2020/12/7 | Accepted: 2021/03/23 | Published: 2022/01/31
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |