BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://journal.zums.ac.ir/article-1-6644-en.html
2- Students Research Committee, School of Dentistry, Isfahan University of Medical Sciences, Isfahan, Iran
3- Dept. of Epidemiology and Biostatistics, School of Health, Isfahan University of Medical Sciences, Isfahan, Iran
4- Dental Research Center, Isfahan University of Medical Sciences, Isfahan, Iran ,
✅ In general, antioxidant supplements may be able to help prevent, treat, and improve the prognosis of this disease, which requires further research in this area.
Global reports consider cancers of the mouth and throat as the sixth most common cancers in the world (1). Oral squamous cell carcinoma (OSCC) usually occurs after the age of 50 in the sixth decade of life. Despite access to the oral cavity during clinical examination, oral cancer is usually diagnosed in advanced stages. The most common cause of this is incorrect initial diagnosis and ignorance by the patient and clinician (2). Several diagnostic tools are developed or will be developing including salivary biomarkers (3). Saliva is a liquid that contains more than 1000 types of proteins, mRNAs, microRNAs, and metabolites that perform a range of biological functions. Cancerous and abnormal tissues of the mouth cause irregular secretion of molecules in saliva that the study of these molecular markers used as a non-invasive method in the diagnosis and treatment of oral diseases (4). Saliva has various defense mechanisms that attack bacteria, viruses, and fungi and also has a protective role against mechanical or chemical attack (5). Nagler and colleagues demonstrated that saliva has antioxidant potential and can inactivate oxygen free radical species such as superoxide (O-2). Production of reactive oxygen radicals plays an important role in human metabolism during physiological changes (6). Oxidants are formed as a natural product of aerobic metabolism, but under pathophysiological conditions, they can produce more rapidly (7). Oxidative stress occurs when the produced oxygen-free radicals exceed their physiological threshold or the body's antioxidant defense system is weak (8). The salivary antioxidant system consists of predominantly water-soluble molecules and enzymes such as uric acid, peroxidase, glutathione peroxidase, catalase, lactate dehydrogenase, glutathione reductase, and aspartate aminotransferase (9). Excessive ROS production or antioxidant deficiency leads to cell damage and ultimately leads to malignant changes. The main target of peroxidation would be unsaturated fatty acids in membrane lipids. The final breakdown products of these lipids are Lipid Hydrogen Peroxide (LHP) and Malondialdehyde (MDA). The final breakdown products of these lipids are Lipid Hydrogen Peroxide (LHP) and Malondialdehyde (MDA). The surface of these final products indicates the rate of lipid peroxidation and is known as a marker of the cell damage caused by free radicals (10). During the body's inflammatory processes, the free radicals produced must be neutralized by the enzymatic antioxidants of glutathione peroxidase (catalase, superoxide dismutase) and non-enzymatic (vitamins A and E and C). One of the suggested reasons for the development of many cancers, such as OSCC, is a disorder in the body's oxidative system (11). Oxidative DNA damage leads to abnormalities in endogenous processes and replication errors, nitrogenous exposure due to oxidative stress, breakage (DNA), and the formation of direct bonds between free radicals, antioxidants, and carcinoma squamous cell (SCC) (12). Normal cells can become malignant cells with oxidative changes (13).
The present study is a systematic review study that performs to compare the changes in salivary antioxidants in patients with squamous cell carcinoma of the mouth with healthy individuals. The whole process of implementation and writing of this study was evaluated based on the PRISMA checklist. To prevent bias, qualitative evaluation and data extraction were performed independently by the original author and executor, and information about this study was searched online from 1980 to June 2020 in foreign databases (PubMed, Science Direct google scholar, Scopus, Web of Science) and the following keywords were obtained:
P: Patients with squamous cell carcinoma
E: salivary antioxidant: Saliva antioxidants
C: Control: Healthy people
O: Output: Antioxidant changes in saliva
The keywords "antioxidant", "saliva", and "squamous cell carcinoma of the mouth" used for internal databases (Iran Medex, Scientific Information Database, Magiran). To combine the results of the studies and to weigh each study, the standard deviation, sample size, and in the case of heterogeneity in the studies, a random-effects model use. Index I2, Q, and TAU2 use to test the studies' heterogeneity and comprehensive meta-analysis (CMA) version 2 software used to analyze the data.
This study was approved with the number of IR.MUI.RESEARCH.REC.1398.702 by Ethics Committee.
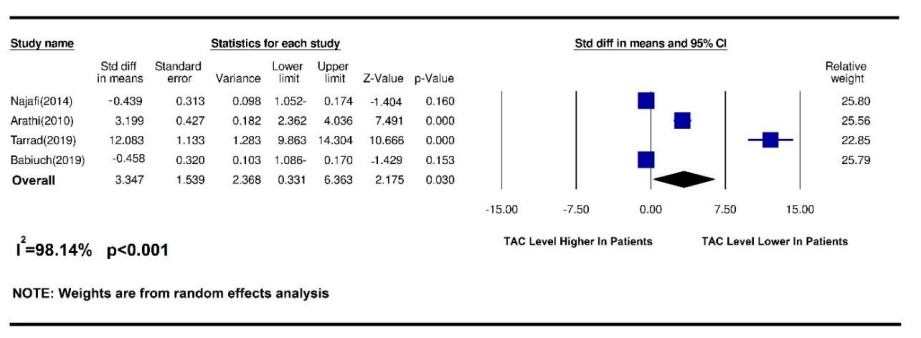
Figure 1. Results of a meta-analysis of changes in salivary TAC index in patients with oral squamous cell carcinoma
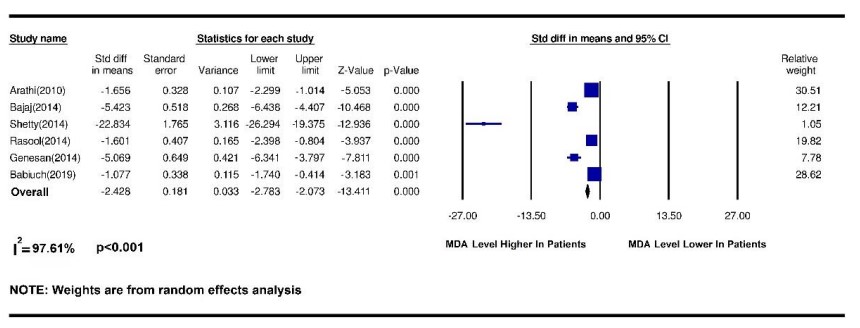
Figure 2. Results of a meta-analysis of changes in salivary MDA index in patients with oral squamous cell carcinoma
Discussion
This study aimed to test the changes in salivary antioxidants in patients with squamous cell carcinoma of the mouth. During a systematic review of databases, 497 related articles were found, of which 26 complete articles were chosen by removing duplicates and screening articles, and were systematically reviewed and meta-analyzed was performed on 10 articles. Among the many antioxidants in saliva, antioxidants such as superoxide dismutase (SOD), glutathione (GSH), and glutathione s-transferase has received the most attention. And in some articles, the total amount of salivary antioxidants or malondialdehyde (MDA) of saliva was measured as an indicator of oxidative stress, which is of great importance due to the group function of antioxidants. To check the number of salivary antioxidants, some researchers used the Oxygen Radical Absorbance Capacity (ORAC) method to measure the antioxidant capacity against oxygen free radicals. Among the articles included in the present study, antioxidant data SOD and GSH as well as total antioxidant capacity (TAC) and MDA were statistically analyzed and antioxidants such as GST as well as ORAC were evaluated due to an insufficient number of complete articles examining these indicators. Statistical analysis was not included in the meta-analysis. Malondialdehyde, the major constituent of which is genotoxic carbonyl, is produced by lipid peroxidation and during arachidonic acid metabolism for the synthesis of prostaglandins. Therefore, the MDA level is used to show oxidation and cell damage caused by ROS and free radicals to tissues (13). In the present study, 6 articles were included in the meta-analysis of the MDA antioxidant index. Arathi et al. in 2009 examined the saliva of people by thiobarbituric acid (TBA) and found that salivary MDA was much higher in patients with OSCC compared with healthy people (14). Metgud et al. Also reported in 2013 that saliva MDA was measured by TBA using a spectrophotometer and that salivary MDA levels were much higher in patients with OSCC than in the healthy group (13). Similarly, Shetty et al. in 2014 examined salivary MDA by thiobarbituric-trichloroacetic acid (TBA-TCA) and found that salivary MDA was significantly different between those with OSCC and those with malignancies compared with healthy people (15). In the present study, by a systematic review of articles and meta-analysis of data, it was shown that salivary MDA was significantly (p <0.001) higher in OSCC patients than in healthy people. Elevated salivary or serum MDA due to oxidative stress supports the hypothesis of increased ROS metabolites in cancer cells compared to non-neoplastic cells and suppression of the antioxidant system by the immune system. Thus, the increase in MDA in patients with leukoplakia and OSCC reflects the response to multiple carcinogens and confirms the increase in oxidative stress and increased lipid peroxidation. Numerous studies have reported increased lipid peroxidation and decreased antioxidant status in patients with SCC (13). Glutathione is the most important intracellular antioxidant and plays an important role in protecting organisms by detoxifying hydrogen peroxidase and reducing OS oxidative stress due to the increased production of free radicals. The main constituent of glutathione is a thiol, which protects the body against the progression of oral cancer by eliminating carcinogens and the effect of lipid peroxidation and maintaining the immune system function by regulating lymphocytic reproduction and mitogenic response. GSH Glutathione is the most abundant non-enzymatic antioxidant that has multiple roles as a substrate for glutathione S G transferase and glutathione peroxidase during lipid peroxidation. Glutathione inhibits ROS and prevents its occurrence, and stores the sulfhydryls necessary for DNA repair. Therefore, the amount of oxidative stress in OS can be managed by evaluating MDA and GSH (13). Regarding the level of salivary GSH index in OSCC, 3 articles were included in the meta-analysis. In 2013, Metgud et al. examined GSH by Beutler et al. and found that salivary GSH levels in healthy people were significantly higher than in patients with OSCC and could be used as a diagnostic marker for precancerous and malignant cavities (13). Babiuch et al. Also found in 2019 that salivary GSH was lower in patients with OSCC than in the healthy group (14). Shetty et al. found in 2014 that GSH levels were higher in the healthy group than in the SCC group (15). In the present study, a meta-analysis of data showed that salivary GSH levels were significantly (p = 0.03) higher in healthy individuals than in patients with OSCC. Contrary to the above findings, Almadori et al. found in 2007 that GSH levels increased in cancer patients (9). Increased salivary GSH in these patients was due to changes in the salivary environment. Active salivary GSH secretion was unrelated to the increase in carcinogens. They concluded that salivary GSH was not a good diagnostic marker for oral squamous cell carcinoma (13). The study of Almadori et al was not included in the meta-analysis due to the lack of accurate reporting of the number of patient groups. Superoxide anion (O-2) is a free radical that has the potential to stimulate oxidative damage and is a common mediator in several biological oxidation processes and cell death mechanisms produced by lysozyme phagocytes after phagocytosis of microorganisms. The enzyme SOD superoxide dismutase acts on these (9). SOD neutralizes this free radical by converting O2 to hydrogen peroxide H2O2 and then by the catalyze and peroxidase activities and converting it to O2 and H2O water (14). To evaluate the effect of salivary SOD index on squamous cell carcinoma, 4 articles were included in the meta-analysis. Singh and colleagues in 2014 obtained similar results using nitroblue tetrazolium (NBT) that salivary SOD levels in patients with OSCC were much lower than in healthy people (15). Shetty et al. Reported in 2014 that salivary SOD levels were lower in people with SCC than in healthy people (16), similarly to Rasool et al. In 2014 (17). In 2019, they found that salivary SOD levels were higher in patients than in the healthy group (18). The reason for this difference in results may be related to the difference in the sample size in these studies. In the present study, by systematically reviewing articles and meta-analysis of data on salivary SOD levels, no significant difference was observed between OSCC patients and healthy people and SOD levels could not be considered different between patients and healthy groups. TAC has all salivary antioxidants, indicating its clinical importance in assessing the antioxidant status of salivary abnormal or pathological conditions. During known antioxidants' evaluation, several antioxidants may remain undetected, so evaluating the overall state of antioxidant activity due to their group interaction may be more valuable (19). To measure the salivary TAC index in OSCC, 4 articles were included in the meta-analysis. In 2014, Najafi et al. examined patients whose biopsies were confirmed by OSCC using saliva centrifugation and concluded that saliva antioxidant levels were significantly higher in patients with OSCC than in the healthy group. Salivary antioxidant levels increase against OSCC. Therefore, prescribing antioxidant supplements can probably be useful and effective in combating free radicals in this field (11). Contrary to the results of this study, Hegde et al. In 2013, by examining the centrifuged saliva of patients with oral cancer and using the phosphomolydbenum method, concluded that the total antioxidant level of salivary antioxidant levels in healthy people was higher than in patients with OSCC (19). This discrepancy may due to the study of oral cancers in general, so this study was not used in the meta-analysis stage. Also, in 2009, Arathi et al., by examining the saliva of patients with OSCC by the method of Kovacevic et al., found that the total antioxidant activity (TAA) in patients with OSCC is much reduced compared to healthy people (14). The latest findings on salivary TAC levels are from the 2019 study by Tarrad et al. And Babich et al., in which Tarrad showed that salivary TAC levels were much lower in people with OSCC than in healthy people. Babich et al. found that salivary TAC levels were much higher in patients with OSCC than in healthy people (18, 20). In the present study, a meta-analysis of data showed that salivary TAC levels were much (p = 0.03) higher in healthy people than in patients with OSCC.
Conclusion
Examining the studies included in this study and the meta-analysis, it was found that in the healthy group the amount of TAC and GSH was higher than the sick group and in the patient group the amount of saliva MDA was higher than the healthy group and there was no significant difference in salivary SOD. They may be used in the prevention, diagnosis, and treatment of patients with squamous cell carcinoma of the mouth.
Acknowledgements
This study has been supported by a grant from Isfahan University of Medical Sciences, Isfahan, Iran.
Financial support and sponsorship
This study has been supported by a grant from Isfahan University of Medical Sciences, Isfahan, Iran.
Conflicts of Interest
The authors of this manuscript declare that they have no conflicts of interest, real or perceived, financial or nonfinancial in this article.
Received: 2021/07/26 | Accepted: 2022/07/4 | Published: 2022/10/10
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |






