BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://journal.zums.ac.ir/article-1-6858-en.html


 , Alireza Mahmoudian *2
, Alireza Mahmoudian *2 

 , Seyyed Meysam Abtahi Froushani1
, Seyyed Meysam Abtahi Froushani1 

 , Abdolghaffar Ownagh1
, Abdolghaffar Ownagh1 

 , Amir Tokmahchi1
, Amir Tokmahchi1 


2- Dept. of Microbiology, Faculty of Veterinary Medicine, Urmia University, Urmia, Iran ,
✅ Therapeutic use of probiotics ameliorated UC by significantly changing the levels of miR-1, miR-99, miR-Let7d and miR-155.
Ulcerative colitis (UC) is categorized as an inflammatory bowel disease (IBD) associated with frequent relapse-remission cycles (1). Etiologically, UC can be triggered by a combination of genetic backgrounds, environmental factors, impaired intestinal microbiota (dysbiosis), allergens, and autoimmune disorders (2).
UC is caused by an intestinal inflammation status established following the release of pro-inflammatory mediators, including cytokines, chemokines, or adhesion molecules (2). Current treatments, including amino salicylates, corticosteroids, immunosuppressants, and antibiotics, may induce side effects (1). Several probiotics, as therapeutics, have been used for prophylactic and treatment purposes with different results in humans and animals (3). Probiotics are found in the guts of most animals and fermented foods and can modify intestinal microbiota and modulate the immune system (4). It has been demonstrated that probiotics interact with protein pattern recognition receptors (PAMP, TLR) and regulate key signalling pathways like NF-κB (5). The infiltration of neutrophils and macrophages into the mucosal tissues of the colon, followed by the release of reactive oxygen species, causes lipid peroxidation, increased permeability of mucosa and blood vessels, increased neutrophil entry into the mucosal tissue, and the development of inflammation (6). Activated oxygen species damage the intestinal wall, cause ulceration, bleeding and diarrhea by affecting the expression of cytokine genes, and enzymes involved in the inflammatory response (7).
The role of micro-RNAs (miRNAs) in the pathogenesis of human IBD has been investigated (8). It has been revealed that changes in the expression of several miRNAs, including miR-21, miR-122a, miR-126, miR-223, miR-146a, miR-150, and miR-155, can modulate immune responses or change the intestinal epithelial permeability (9). MicroRNA molecules are sequences of 18-22 nucleotide, which can regulate cellular activities by destroying or inhibiting the expression of the target mRNA, and play a crucial role in various human diseases, such as cancer, neurodegenerative disorders, diabetes, heart hypertrophy, and respiratory diseases (10). MiRNAs play an essential role in innate and acquired immunity by regulating the production of MEPs (megakaryocyte-erythroid generators) and GMPs (granulocyte-macrophage generators) from CMPs (common myeloid generators) as well as terminal myeloid cells (dendritic cells, macrophages, platelets, and red blood cells). miRNAs regulate the innate immune response through PAMP-dependent activation of NF-Κb, MyD88, MAPK, and jak/STAT followed by inflammatory cytokines (IL-1, IL-6, TNFα, IFN-γ, and IFNβ) and antigen-presenting genes. miRNAs are involved in numerious developmental stages of lymphopoiesis, including B cell differentiation and different types of T cells (11). miRNAs are essential modulators of intestinal homeostasis, immune cell response, and autophagy by directly or indirectly disrupting the process of vesicle enlargement. They are involved in intestinal homeostasis by regulating epithelial integrity (29).
Each miRNA can modulate multiple mRNAs, and a single mRNA can usually be targeted by several miRNAs (18). It is estimated that over 30% of protein-coding genes are regulated by miRNAs (19). Generally, miRNAs are involved in almost all cellular activities, including metabolism, development, and cell cycle under healthy and pathological conditions (20).
To understand the extent of beneficial effects of probiotics on the acid-induced UC in rats, as an animal model, we sought to investigate the ameliorating effects of L. acidophilus on clinical signs and the intestinal pathogenesis by focusing on its immunomodulatory properties and determining the role of miRNAs in the UC pathology.
Animal experimentation
Twenty Wistar rats were purchased from the animal breeding centre of the Faculty of Veterinary Medicine of Urmia University. Ethical considerations regarding experiments on animals were considered following the Helsinki Convention, and the study was approved by the Ethics Committee of our faculty (Ethical code:IR-UU-AEC-2129/PD/3). The animals had rest for seven days prior to experiments to adapt to the environmental conditions. The rats were randomly divided into four groups of five in each, including control group, probiotic receiving rats, acid-induced colitis (UC) group, and UC rats receiving probiotic group.
Induction of ulcerative colitis
The animals fasted for 24 hours, while they were only allowed to drink water. To induce colitis, the rats were briefly anesthetized using a ketamine-xylazine mixture and 1 ml of acetic acid (4%) was injected into their rectum using a Foley catheter, as explained previously (12).
Preparation and administration of probiotic
L. acidophilus ATCC4356 was cultured in the MRS broth for 24 hours, followed by the MRS agar culture for 48 hours at 37 °C. The rats were fed with 500 μl of dilutions containing 3×108 CCU/ml of the probiotic bacteria.
Evaluation of disease activity index (DAI)
Disease activity index (DAI) was determined by the following scoring system: Weight loss: 3=< 20%, 2=10-19%, 1=1-9%, 0=negative. Stool consistency: 3Diarrhea 2=very soft, 1=soft, 0=normal. Blood in the faeces: 3=black, 2=dark red, 1=red, 0=negative. The sum of the three items per day was calculated for each rat. DAI were monitored for ten days.
Tissue sample preparation
On day 10, the rats were sacrificed using intraperitoneal injection of a mixture of 10% ketamine (50 mg/kg) and 2% xylazine (10 mg/kg). The intestine, spleen, kidney and liver were removed and then weighed. A small section of samples was kept in 10% formalin for immunohistochemistry (IHC) and Hematoxylin and eosin (H&E), as explained somewhere else (13). A small portion of tissues was stored at -80 °C for the total RNA extraction. The remaining tissues were homogenized in 1 ml of normal saline using a homogenizer for further experiments.
qPCR assays
Samples were homogenized using 3-way tabs, and a total volume of 50 μl of the total RNA was extracted using a phenol-chloroform-based commercial kit (YTzol pure RNA extraction kit YektaTajjhiz Azma Co., Iran), according to the manufacturer’s instructions.
The concentration and purity (260/280 ratio) of RNAs were measured using a NanoDrop spectrophotometer. To prepare cDNA, DNA contaminations were first removed using RNase-free DNAase 1 (YektaTajhizAzma Co.), according to the manufacturer’s instructions. To determine the levels of mRNAs of the cytokines, 500 μg of RNA were reversely transcribed using an AccuPower NanoDrop ® cDNA synthesis kit (Bioneer, Daejeon, Korea), and then the cDNAs were refrigerated at 4 °C. To amplificate miRNAs, 500 ng of the total RNAs were reversely transcribed using a microRNA PCR miRCURY LNA Universal RT system (Qiagen, Germany), according to the manufacturer’s instructions. To perform qPCR assays, reaction mastermixs were prepared in a final volume of 20 μl using a Bioneer SYBR Green mastermix (Bioneer, Korea). Oligonucleotide primers for each cytokine gene were designed according to the basics explained previously (14) and manufactured by Integrated DNA Technologies (IDT, Australia). The variations in Ct values were normalized using HRPT and sno234 snRNA internal control genes for cytokines and miRNA experiments, respectively. The levels of the expressions were calculated using the 2-ΔΔct method (14), as explained previously. Amplifications were performed using an Applied Biosystems StepOne™ Real-time PCR thermocycler. The sequences of the primers used in the present study were as follows: TNF-α-R: CTTGATGGCAGAGAGGAGG, TNF-α-F: CTCTTCAAGGGACAAGGCT; IL-1β-F: CAGCTGGAGAGTGTGGATC, IL-1β-R: TGCTGATGTACCAGTTGGG; IL-6-F: GCCCTTCAGGAACAGCTATGA, IL-6-R: TGTCAACAACATCAGTCCCAAGA; IL-10-F: TAAGGGTTACTTGGGTTGCC, IL-10-R: ATGCTCCTTGATTTCTGGGC; IFN-γ-F: TGGAGGAACTGGCAAAAGG, IFN-γ-R: CGCTTATGTTGTTGCTGATGG; miR-1: TGGAATGTAAAGAAGTATGTAT; miR-99a-5p; AACCCGTAGATCCGATCTTGTG; miR-155-5p: TTAATGCTAATCGTGATAGGGGT; Let-7d: CTATACGACCTGCTGCCTTTCT; USLP-R: CACATTCTTCCGTCCTCGTGC.
Biomedical assays
Cyclooxygenase-2 (COX-2) protein expressions, as an index for the gastrointestinal mucosal integrity, were assessed by Cox-2 biotinylated primary antibodies (Elab sciences, USA, Cat number: E-AB-62884) using the immunohistochemistry (IHC) staining method as explained previously (13). Tissue slides were analyzed using the Hematoxylin and Eosin (H&E) staining method. The activity levels of myeloperoxidase, Malondialdehyde, and Nitrite oxide were determined with an ELISA reader (Dana ELISA Reader 3200, Iran), using a Nampox ™ Myeloperoxidase (MPO) Activity Assay Kit, a Nalondi ™ Lipid Peroxidation (MDA) Assay Kit, and a Natrix ™ Nitric Oxide (NO) Assay Kit, all from Navand Salamat Co., Iran, and according to the manufacturer’s instructions (15).
Statistical analysis
The non-parametric and normality of the data were analyzed using Kruskal-Wallis Kolmogorov-Smirnov methods, respectively. The variances between the groups were determined using the one-way ANOVA method (SPSS software version 26.0, SPSS, USA), and the P values ≤0.05 were considered statistically significant.
As Figure 1 shows, rats with colitis that were treated with probiotics had an improved DAI score, compared with those in the UC group, and the rats in the probiotic and control groups had the same DAI scores.
Figure 1. A) Disease Activity Index (DAI) scores during the 10-days course of the experiments. B) Mean DAI. DAI was significantly different between the groups that were labelled by capital “A”, “B” and “C” and no significant differences were found between two groups that labelled by “A” (the Control and the Probiotic groups).
As Figure 2 depicts, in the control and probiotic receiving groups, no bleeding or weight loss was found, stool consistency was normal, and no lesions were detected in intestinal tissues. Rates receiving both acid and probiotics had clinical signs, and their intestinal tissues were congested. However, during the study, the rats had less bleeding, better stool consistency, and increased body weight, compared with the acid-induced colitis (UC) group. Administration of acetic acid in the rectum induced diffuse inflammatory reactions, migration of neutrophils and lymphocytes, and ulceration in the intestinal epithelium (Figure 2).
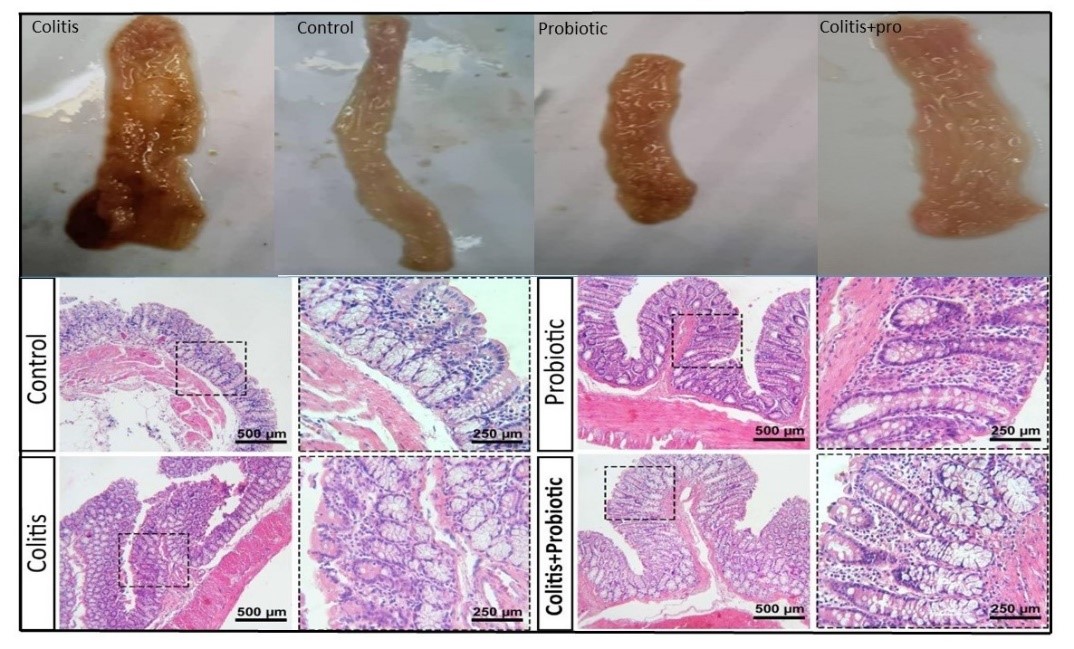
Figure 2. Microscopic and microscopic assessment of the colitis in rats. Gross assessment of colons: control group, colitis group, colitic rats treated with L. acidophilus group, and probiotic group. Light microscopic assessment (H&E staining) of the mucosal layer of the intestine in the control and the colitis rats treated with L. acidophilus group; infiltration of immune cells was found much less to in the colitis.
COX-2 expression was increased in the UC group compared with the control group. In rats receiving both acid and probiotic, the COX-2 expression decreased compared with the UC group; however, no difference was observed between the control and probiotic groups (Figure 3).

Figure 3. Analyses of the pixel-based intensities of the COX-2 protein (brown-stained). The graphs are created using the Image J software (National Institutes of Health, USA) from 2530µm × 2530 µm cross-sections. The results are presented in Mean ± SD of brown pixels/total pixels intensity; the mean of the pixel-based intensities was calculated from 3 readings for each animal in each group. The expression of COX-2 protein decreased significantly in the UC rats treated with L. acidophilus compared to acid-induced UC rats (P≤0.05).
As Figure 4A shows, the levels of the expression of TNF-α, IFN-γ, IL-10, IL-1β, and IL-6 cytokines in the colitis group, compared with control rats, were significantly increased. The expression of IL-1, IL-6, IL-10, IFN-α and INF-γ was significantly decreased in rats treated with probiotic (around 3.7, 5.1, 1.7, 1.9 and 1.5-fold increase compared with healthy control rats, respectively).
The colitic rats treated with probiotic had significantly lower levels of mRNA for IL-1, TNF-α and IFN-γ compared with the acid-induced colitis group (Figure 4 A). The levels of IL-1 and TNF-α mRNA were lower, and the level of IFN-γ was higher in UC rats receiving probiotics compared with healthy rats receiving probiotic; however, these changes were not statistically significant. The expression levels of IL-6 and IL-10 were lower in the acid-induced colitis, probiotic and acid and probiotic treated groups than in the control group (Figure 4 A).
The expression levels of miR-1, miR-99, miR-155, and Let-7 were determined using qPCR assays. As Figure 4 B depicts, all four investigated miRNAs expression levels had significant changes in colitic rats. The miR-1 expression was significantly decreased in probiotic-treated rats (around a 2-fold decrease). The expression levels of miR-155, miR-99, and miR-let7d were decreased in probiotic-treated rats (around a 0.2-fold increase compared with colitis rats, respectively).
The expression of miR-1 in the control group decreased significantly. The expression of miR-1 in the treatment and probiotic groups decreased significantly (Figure 4 B). The expression of miR-1 in the colitis group slightly increased compared with the control group; however, it decreased significantly in both probiotic-only and UC treated with probiotic groups (approximately a 2- and 4-fold decrease, respectively, compared with the control group) (Figure 4 B).
Figure 4 B also shows that the expression of miR-99a in the colitis group was slightly higher, and in both probiotic-only and UC treated with probiotic groups, the expression was slightly less than that of the control group.
The expression levels of let-7d were downregulated in all three groups compared with the control rats, and this reduction was more obvious in the probiotic-only group (approximately a 1-fold reduction) than in UC rats receiving probiotics (around a 0.2-fold reduction). Additionally, the levels of miR-155a had a statistically significant but very slight increase in all three groups (Figure 4 B).

Figure 4. Fold-change differences in the levels mRNA of cytokine genes and miRNAs between the groups. The differences in the levels of the expression of mRNA and miRNAs are normalized using HRPT mRNA and sno234 miRNA, as internal control genes, and are calculated using the 2-ΔΔCt equation. The differences are shown based on the normalized data to the control group. The positive and negative bars had higher and lower expression levels compared to the control group, respectively.
The analysis of the changes in the levels of biochemical parameters in the colon indicated that the levels of MPO, malondialdehyde and nitric oxide in rats with colitis were significantly increased compared with healthy rats. Meanwhile, the administration of probiotics significantly reduced the activity levels of MPO, NO and malondialdehyde compared with acid-induced colitis rats. Statistically, the changes in the biochemical parameters were not different between the control and probiotic groups (Figure 5).

Figure 5. Assessments of the biochemical activities in the intestinal specimens in different groups; (A) Nitric oxide, (B) myeloperoxidase, and (C) malondialdehyde activities decreased in the acid-induced colitis group and the UC rats treated with L. acidophilus. Data are shown as mean ± SEM. Data was significantly different between the groups that labelled by capital “A”, “B” and “C” and no significant differences were found between two groups that labelled by “A” (the Control and the Probiotic groups).
Discussion
It has been shown that changes in the intestinal microflora can deteriorate the pathogenesis of IBD (16). To ameliorate the symptoms, therapeutic effects of several probiotics, including Escherichia coli Nissle, Lactobacillus casei, Bifidobacterium lactis, Lactobacillus acidophilus, and E. faecalis have been investigated in people suffering from IBD (17).
The mechanisms by which probiotics can fulfil their functions are still under investigation. In recent years, the role of microRNAs in the pathogenesis of diseases and their response to different treatments have been studied.
In the current study, we aimed to analyze changes in the expression levels of selected microRNAs following the administration of L. acidophilus in acid-induced ulcerative colitis.
As mentioned (17), the intestinal administration of 4% acetic acid causes diffused epithelial hyperaemia, bleeding, erosion, and focal ulceration, and increases mucosal permeability and leukocyte infiltration. Following colitis induction and administration of probiotics, the levels of MPO, NO, MDA, IL-1, IL-6, IFNγand TNF-α were changed. Acetic acid induces oxidative stress, activities TLR-4 / NF-κB / MAPK signaling pathways, and reduces the IL-10 / JAK1 / STAT3 response. Acetic acid also increases the expression of MyD88 in colonic tissues, and p-NF-κB p65, p-JNK, p-ERK, and p-P38 proteins. In the dextran sodium sulfate (DSS)-induced colitis, the destruction of the Muc2 mucosal barrier increases intestinal permeability and crypt hyperplasia and triggers immune responses, including the expression of cytokines such as TNF-α, IL-1β, and IL-6 (21). It has been demonstrated that in animals with DSS-induced UC, the administration of Lactobacillus lactis can alleviate the intensity of the inflammation (22).
It has been found that DSS-induced colitis is associated with increased neutrophil infiltration, and increased activity levels of lipid peroxidase, myeloperoxidase, nitric oxide synthetase, and malondialdehyde. (17).
Treatment with probiotics increased numerous inflammatory markers, improved weight gain, and prevented diarrhea and rectal bleeding in rats with ulcerative colitis. Our clinical data demonstrated. The activity levels of COX-2, MDA, and NO were determined to assess the extent of the pathological damage caused by the induction of colitis. During the inflammation, the family members of COX enzymes catalyze the conversion of arachidonic acid to prostaglandins, which play a key regulatory role in gastrointestinal motility and functions (23). MDA increases during the oxidative stress, and its production is associated with the breakdown of unsaturated fatty acids and thus, is considered an indicator for the levels of lipid peroxidation (18). Nitric oxide is also a signaling molecule involved in several physiological and pathological processes (24). The expression of cytokines in the probiotic group was slightly reduced compared with the control group.
Unlike other cytokines, IFN-γ was reduced in the treated group, compared with probiotics; however, these changes were not statistically significant for none of them. Treatment with Lactobacillus acidophilus probiotic reduced cell infiltration and improved inflammation in rats with colitis.
The mucosal barrier is composed of a layer of highly distinct AJ, TJ and desmosome epithelial cells, and plays a key role in maintaining the normal functions and homeostasis of the intestine. Multiple signaling proteins, including protein kinases, protein phosphatases, and G proteins, regulated the functions of TJ cells. AJ cells act as a permeable barrier, and their function can be disrupted by ROS, cytokines, and pathogens by manipulating the protein kinases (PKC). Probiotics can help to maintain the functions of TJ and AJ cells.
Mucosal layer, as the first innate defense barrier, protects the epithelium from commensal bacteria. In response to the pathogen, goblet cells can increase the secretion of different mucus (MUC) proteins to neutralize the bacteria. MUC1 enhances the viability of intestinal epithelial cells and their resistance to chemical irritants by activating the EGFR/Akt/c-FLIP/COX2 cascade. MUC2 is typically expressed in organs like the large intestine and has a protective role. MUC4, a tyrosine kinase receptor ligand with anti-apoptotic effects, mediates PI3K/Akt/MAPK and c-Src/FAK kinase family pathways to regulate cell proliferation and metastasis (25). It has been demonstrated that miRNAs can modulate the expression of various mucin family members.
Previously, it was revealed that Lactobacillus plantarum could activate the TLR2 pathway and increase the MUC3 production (26).
MiRNAs affect the intestinal barrier function by regulating TJ. Following intestinal inflammation and increasing the expression of TNF-α and interferons, the expression of miR-155 increases, which in turn inhibits the RhoA protein, thereby reducing the expression of zonula occludins-1 (ZO-1) and E-cadherin as the two major proteins of AJC components (27).
The levels of miR-155 were significantly higher in UC rats compared with the control group. Administration of probiotics reduced the levels of miR-155, as well as TNF-α, IL-6, IL-12, IL-17, and IFN-γ. It has been shown that miR-155 regulates FOXO3a and SOCS1 (28), enhances Th1 and Th17 cell growth, and facilitates Th17 cell formation through cytokines released by dendritic cells that play a vital role in the IBD pathology. miR155 maintains the homeostasis of Treg cells by targeting the cytokine signaling suppressor one via the IL2 signaling pathway. They also regulate the performance of B cells (29). MiR-155 is involved in the activation of the LPS / TNF-α pathway, suppresses the SOCS-1 signaling pathway, and negatively regulates the dendritic cell antigen presentation capacity (19, 29).
It has been demonstrated that miR-155 can contribute to the pathogenesis of colitis by significantly reducing the levels of SHIP-1 mRNA (30).
MiR-99a was found to be differently expressed in UC rats. The miR-99 family as potential tumor suppressors regulates the DNA damage response (31).
The levels of let-7d were found to be differentially expressed in UC rates treated with L. acidophilus compared with the control group. The let-7 family members are highly conserved across diverse animal species from worms to humans and play crucial roles in regulating cell proliferation and differentiation (32), and the NF-κB pathway. Furthermore, let-7f has been found to be differentially expressed in the intestinal tissue of active UC patients (33). The lower levels of let-7d miRNA caused by probiotics in our study may suggest that let-7d can contribute to the pathogenesis of colitis, and the healing effect of probiotics may be partly concerned with the reducing capability of the let-7d levels in experimental animals.
Interestingly, miR-1 was expressed higher in UC rats, while it is expressed primarily in myocardial cells, and its level reduces following myocardial infraction. miR-1 can induce differentiation of myoblast cells by downregulating Notch3 and reduces in several types of cancer. miR-1 acts as a tumor suppressor by interacting with multiple oncogenes such as MET oncogene and oncogenic pathways (34). Our data indicate that the ameliorating properties of probiotics may be associated with the regulation of the pathways involved in the pathogenesis excreted by miR-1.
Conclusion
Our findings contribute to the current knowledge of the putative roles of miRNAs in the pathogenesis of the UC exerted by the beneficial properties of L. acidophilus, as evidenced by the amelioration of clinical signs and pathological characteristics in rats suffering ulcerative colitis. The identification of differentially expressed miRNAs and the understanding of their molecular mechanisms contribute to determining the pathophysiology of UC, discovering new diagnostic biomarkers, and developing new therapeutics.
Acknowledgements
None.
Conflicts of Interest
None.
Received: 2022/01/18 | Accepted: 2022/07/13 | Published: 2022/12/12
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |




