BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://journal.zums.ac.ir/article-1-5966-en.html


 , Shima Shahyad2
, Shima Shahyad2 

 , Fariba Namdar1
, Fariba Namdar1 

 , Ali Noroozzadeh1
, Ali Noroozzadeh1 

 , Zahra Bahari1
, Zahra Bahari1 

 , Mohammad Taghi Mohammadi *3
, Mohammad Taghi Mohammadi *3 


2- Neuroscience Research Center, Baqiyatallah University of Medical Sciences, Tehran, Iran
3- Exercise Physiology Research Center, Baqiyatallah University of Medical Sciences, Tehran, Iran. ,
✅ According to our findings, fullerene C60 nanoparticle could reduce oxidative stress damage in diabetic condition in the rat testicular tissue probably through potentiating the antioxidant defense system.
Male reproductive dysfunction is one of the main problems of diabetes mellitus (DM) that is observed in diabetic men (1). This disease deleteriously impairs the functions of male reproductive system in several parts, including spermatogenesis, sperm maturation, fertility capability, and sexual function (1, 2). A remarkable change in male sexual hormones is another problem that occurs in diabetic men due to alteration in the reproductive endocrine system (3). In this regard, a decrease in testosterone secretion and reduction of serum testosterone levels as well as decreased serum levels of LH and FSH has been demonstrated in the animal models with DM (3, 4). Impairment in spermatogenesis is another complication in the diabetic individuals that has been observed as oligospermia and azoospermia (5). It appears that several pathogenic mechanisms are involved in reproductive dysfunction in diabetes, including decreased sexual hormone levels, occurrence of oxidative stress, activation of degenerative and apoptotic signals in the testes, and impaired energy metabolism pathways (1, 3, 6). According to the evidence, oxidative stress damage is one of the key participants in developing reproductive and testicular dysfunction in diabetic men, which consequently leads to male infertility (6, 7). Overproduction and accumulation of free radicals, particularly superoxide anions, have been reported in the testicular tissue in diabetes (6). Hence, due to a higher amount of polyunsaturated fatty acids in germ cells, these cells are more susceptible to oxidation by free radicals than other cells in the testicular tissue (7).
Fullerene C60 nanoparticle is the third allotrope of carbon atom with the diameter of 0.72 nm, and a remarkably stable structure that is extensively considered for biomedical usage due to their beneficial biological functions (8, 9). These types of nanoparticles have the potent antioxidant properties (several hundred-fold higher than common endogenous antioxidants) due to distinct redox chemistry and organization of vastly delocalized π double bonds (10). According to the numerous reports, C60 fullerenes and their derivatives show tremendous protection versus oxidative stress damage without causing acute or sub-acute toxicity (9, 11). Hence, due to the potent antioxidant activity of fullerenes, they are used as therapies in a wide range of pathological states (8). In this regard, the potential hepatoprotective activity of fullerene C60 against oxidative stress has been reported in the liver of diabetic rats probably through potentiating the antioxidant defense system (12). Fullerene C60 derivatives also improve oxidative stress and decrease cell cytotoxicity developed by photo (13). So, daily and long-term treatment with fullerene C60 nanoparticles prolongs survival in an experimental model of CCl4 intoxication in rats (14). Likewise, the neuroprotective activity of fullerene C60 against cerebral ischemia/reperfusion injuries has been demonstrated through improving oxidative stress damage (15). Additionally, administering fullerene C60 in rats could protect the liver against cyclophosphamide-induced hepatotoxicity (16).
Since the various beneficial properties of fullerene C60 nanoparticles were reported in the literature, the effects of these nanoparticles on the markers of oxidative stress damage in testicular tissue during diabetic condition have remained to be determined. Therefore, the present study assessed the effects of oral repeated administration of fullerene C60 nanoparticles on the markers of oxidative stress damage in the testes of streptozotocin-induced diabetic rats.
Animals
The present study was an interventional-experimental study. To perform the current study, the standard guidelines, presented by Ethics Committee of Baqiyatallah University of Medical Sciences, were considered for all experimental procedures, including animal anesthesia, animal killing, blood sampling, animal caring and using. Male Wistar rats were kept in a standard condition with 12 hours light/dark cycles, humidity of 40‒60% and temperature of 22‒24ᵒC, and ad libitum access to a standard rat chow and water.
Fullerene C60 Nanomaterial
In the present study, fullerene C60 nanoparticle was synthesized in Sharif University of Technology (Iran) by arc heating of graphite and laser irradiation of poly aromatic hydrocarbon (17). In this method, which is the main route for fullerenes synthesis, great quantities of fullerene C60 nanomaterials are produced with purity above 90%. Sesame oil was used as fullerene C60 vehicle, and this nanoparticle was orally administered by a gavage tube at the lowest dose (1 mg/kg, daily) based on solubility of fullerene C60 in the oils and to avoid any tissue toxicity according to the previous studies (14, 18).
Diabetes Induction
Rats were allowed one week to acclimatize before diabetes development, and then diabetes was developed by an i.v. Injection of 45 mg/kg streptozotocin (STZ, Sigma Aldrich, USA) through the tail vein (19). Same volume of saline (as vehicle of STZ) was administered in the non-diabetic animals. Confirming of diabetes was done by determination of blood glucose level and the rats with high blood glucose (>450 mg/dL) were selected as diabetic animals.
Experimental Protocols
To perform the current study, 32 male Wistar rats, aging 8‒10 weeks old and weighting about 220±20 gr, were assigned to four groups (each group, n=8) as follows: two control (normal and diabetic) and two treated (normal and diabetic) groups. The first group was considered as normal (control group) and the animals in this group received sesame oil (fullerene vehicle) for 8 weeks, daily and orally. The second group was considered as fullerene-treated group and during the study, the rats in this group received daily fullerene C60 nanoparticles (1 mg/kg), dissolved in sesame oil, by gavage for 8 weeks. The third group used as control diabetic group and the animals of this group, like the normal group, received sesame oil (fullerene vehicle) for 8 weeks, daily and orally. The fourth group was considered as a fullerene-treated diabetic group and these rats received daily fullerene C60 nanoparticles (1 mg/kg), dissolved in sesame oil, by gavage for 8 weeks during the test. Treatment of diabetic rats started on day 5 after STZ injection and continued for 8 weeks. Blood samples (500μL) were obtained on day 5 after STZ injection and the end of the study under light anesthesia from the animal tail. Then, the blood samples were centrifuged (4500×g) and the separated serum was used for assessing the serum glucose.
Tissue Preparation and Determination of Protein Levels
At the termination of the study, the rats were deeply anesthetized and the testes were removed. After homogenizing the testes using phosphate buffered saline (PBS), the samples were centrifuged for 15 min (14000 g and 4ᵒC). Afterwards, the supernatants were removed to be used for assessing the markers of oxidative stress damage and protein concentration by the Bradford method.
Determination of the Markers of Oxidative Stress Damage in the Testes
Determination of malondialdehyde content: To determine the malondialdehyde (MDA) content in the tissue homogenate, first protein was precipitated by mixing 0.5 mL of the tissue sample with 1.5 mL trichloroacetic acid (TCA) 10%. The sample was vortexed, and after incubation for 10 h (room temperature) supernatant was separated. After mixing 2 mL of thiobarbituric acid (0.67%) with 1.5 mL of supernatant, the sample was placed in boiling water (30 min) using a sealed tube. Then, 1.25 mL of n-butanol was added to sample, and after cooling, the solution was again vortexed. After centrifuging the solution for 5 min at 2000 g, the supernatant was removed to record the absorbance of solution at wave length of 532 nm. At the end, 1, 1, 3, 3-Tetraethoxypropane was used to obtain a standard curve, and MDA content of the testes was computed as nMol/mg protein.
Determination of the Activity of Catalase: To determine the catalase (CAT) activity in the testes homogenates, firstly, the reaction mixture was prepared, which comprised the homogenated solution (0.1 ml) and potassium phosphate buffer (0.85 ml, 50 mM, pH 7.0), and the solution was allowed to be ready for 10 min at room temperature. After mixing the sample with H2O2 (0.05 ml) 30 mM, which was produced in 50 mM potassium phosphate buffer and pH=7.0, the reaction was allowed to be done. At the final stage, a decrease in the solution absorbance was read at the wave length of 240 nm for 3 min, and the catalase activity of the testes was computed in U/mg protein (20).
Measuring superoxide dismutase (SOD) activity of the testes: In the present study, the activity of SOD in the testes was measured by the Winterbourn method (21). The activity of SOD enzyme was measured by mixing potassium phosphate buffer 0.067 M (pH 7.8) with EDTA 0.1 M, and a solution comprising nitroblue tetrazolium (NBT) 1.5 mM, riboflavin 0.12 mM, sodium cyanide 0.3 mM, and 0.1 mL sample. Then, the sample was incubated in room temperature for 12 min, the solution absorbance was read at wave length of 610 nm for 5min. One Unit was the enzyme content that could produce 50% inhibition, and ultimately the activity of SOD enzyme in the testes was considered in U/mg protein.
Measuring the content of glutathione (GSH): In the present study, the Tietz method was applied for measuring the content of GSH in the testes (22). In the beginning, sulfosalicylic acid (5%) was applied for precipitation of protein in the homogenated solution, and then supernatant was removed after centrifuging the sample for 10 min (2000 g). Afterward, to determine the content of GSH in the samples, 100 mL of 0.04% 5,50-dithiobis-(2-nitrobenzoic acid) (DTNB) in 0.1% sodium citrate and 800 mL of 0.3 mM Na2HPO4 was mixed with 100 µL protein-free supernatant. At the final stage, the solution absorbance was read after 5 min at wave length of 412 nm, and the content of glutathione in the testes was computed in nMol/mg protein.
Statistical Analysis
SPSS software 21 (SPSS Inc., Chicago, IL., USA) was applied to analyze for data of the current study. Thus, one-way ANOVA and Tukey’s post-hoc tests were applied for the statistical comparison of the between-group data. All data were expressed as mean±SEM, and a P-value<0.05 was considered as a significant difference.
Effect of Fullerene C60 on Blood Glucose Level
As illustrated in Figure 1, the level of blood glucose was 125±11 mg/dL for the normal rats at the end of the study. Inducing diabetes by STZ injection significantly increased blood glucose level of diabetic animals (431±28 mg/dL). Blood glucose level of the treated diabetic rats was 417±63 mg/dL, which did not change by fullerene C60 treatment. Treatment with this nanoparticle did not affect the value of blood glucose in normal animals either (122±6 mg/dL).
Effect of Fullerene on Weight Index of Testis
Weight index of the testis (g/kg) is illustrated in Figure 2 separately for right and left testes. According to Figure 2A, weight index of right testis for normal group was 4.85±0.27 g/kg. Treatment with fullerene C60 did not notably affect weight index in the right testes of treated normal animals (4.99±0.23 g/kg). Induction of diabetes by STZ injection significantly increased weight index of right testis in diabetic animals (7.27±0.43 g/kg). Fullerene C60 administration significantly decreased weight index of right testis in treated diabetic rats (5.68±0.47 g/kg). Alterations of weight index of left testis (Figure 2B) in different groups for this study were similar to right testis. Diabetes induction significantly increased weight index of left testis in diabetic animals (7.50±0.44 g/kg) compared to the normal group (5.02±0.2 g/kg). Treatment with fullerene C60 significantly decreased weight index of left testis in treated diabetic rats (5.86±0.41 g/kg).
Effect of Fullerene C60 on the Markers of Oxidative Stress Damage
Effect of fullerene on malondialdehyde (MDA) level: The MDA level in the rat testis is shown in Figure 3. Rats with STZ-induced diabetes exhibited a significantly increased testis MDA level compared to the normal rats (P<0.001). Fullerene C60 administration significantly decreased MDA level of the rat testis in the treated diabetic animals compared to the diabetic group (P<0.001). However, administering fullerene C60 in the normal rats did not affect MDA level of the testes.
Effect of fullerene C60 on the activities of catalase and superoxide dismutase: As illustrated in Figure 4A, the rats with STZ-induced diabetes showed a significantly decreased CAT activity in the rat testes compared to the normal group (P<0.05). Treatment with fullerene C60 prevented the considerable reduction of CAT activity in the testes of the treated diabetic animals compared to the diabetic group. However, treating the normal rats with fullerene C60 did not change CAT activity of the rat testis.
SOD activity of the rat testis is shown in Figure 4#f4B. Inducing diabetes did not affect the activity of SOD enzyme in the rat testes among the diabetic animals compared to the normal rats. Treatment with fullerene C60 nanoparticle did not affect SOD activity in the diabetic animals either. However, treating the normal rats with fullerene C60 significantly decreased the activity of SOD enzyme in the testes of the normal animals (P<0.05).
Effect of fullerene C60 on glutathione level: The GSH content of rat testis is shown in Figure 5. Induction of diabetes did not significantly change GSH content of rat testis in diabetic animals compared to normal rats. Treatment with fullerene C60 did not significantly change GSH content of rat testis in treated normal and diabetic rats either.
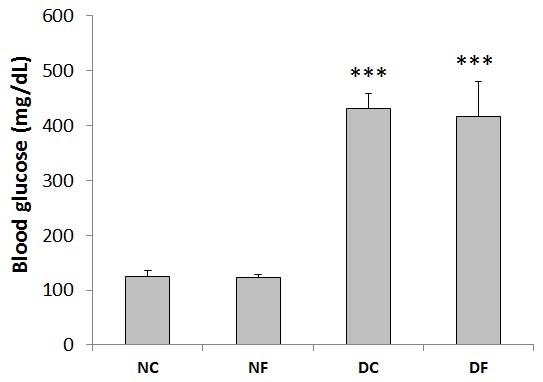
Figure 1. Blood glucose levels of rats at the end of the test (NC; normal control group, NF; fullerene-treated normal group, DC; diabetic control group, DF; fullerene-treated diabetic group). All values are presented as mean±SEM. ***, P-value<0.001 compared to normal group.
![Figure 2. Right (A) and left (B) testis index [tissue weight (g)/body weight (kg)] of rats at the end of the test (NC; normal control group, NF; fullerene-treated normal group, DC; diabetic control group, DF; fullerene-treated diabetic group). All values are presented as mean±SEM. **, P-value< 0.01 compared to normal group; †, P-value<0.05 compared to diabetic group.](./files/site1/ghorbanzadeh_6b9d1c9/images/JAMBR_Volume28_Issue133/03-02.JPG)
Figure 2. Right (A) and left (B) testis index [tissue weight (g)/body weight (kg)] of rats at the end of the test (NC; normal control group, NF; fullerene-treated normal group, DC; diabetic control group, DF; fullerene-treated diabetic group). All values are presented as mean±SEM. **, P-value< 0.01 compared to normal group; †, P-value<0.05 compared to diabetic group.

Figure 3. The MDA levels of rat testis at the end of the test (NC; normal control group, NF; fullerene-treated normal group, DC; diabetic control group, DF; fullerene-treated diabetic group). All values are presented as mean±SEM. ***, P-value<0.001 compared to normal group; †††, P-value<0.001 compared to diabetic group.
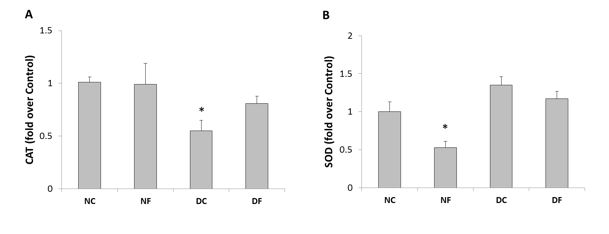
Figure 4. The activity of catalase (CAT; A) and superoxide dismutase (SOD; B) in the rat testis at the end of study (NC; normal control group, NF; fullerene-treated normal group, DC; diabetic control group, DF; fullerene-treated diabetic group). All values are presented as mean±SEM. *, P-value<0.05 compared to normal group.
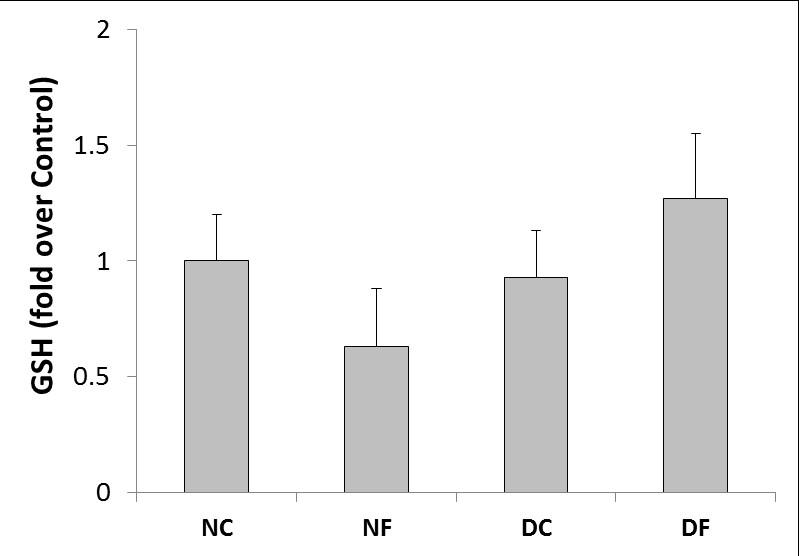
Figure 5. The glutathione (GSH) content of rat testis at the end of the test (NC; normal control group, NF; fullerene-treated normal group, DC; diabetic control group, DF; fullerene-treated diabetic group). The values are presented as mean±SEM.
Discussion
According to previous studies, DM causes testicular dysfunction and development of oxidative damage in male rat testis (1, 4). Since fullerene C60 was proposed as an excellent antioxidant for protecting against oxidative stress-induced tissue damage (23), we decided to assess the effects of fullerene C60 nanoparticle on the markers of oxidative stress and antioxidant system in the STZ diabetes-induced in testicular tissue of diabetic male rats. The results of the present study indicated that fullerene C60 had a capability to decrease MDA levels (the main index of oxidative stress) of the testes in diabetic rats without a considerable change in antioxidant capacity of testicular tissue. Administration of fullerene C60 nanoparticle during diabetes also improved right and left testis index in treated diabetic rats. However, blood glucose level was not changed in the fullerene-treated normal or diabetic animals. In this regard, Nedzvetskii et al., demonstrated that administration of hydrated С60 fullerene did not affect the blood glucose level of rats with experimentally-induced diabetes (24). As yet, no study has reported the alteration of blood glucose level by administration of fullerene C60 nanoparticle and its derivatives.
In the present study, STZ-induced diabetes increased the MDA levels (as a valuable parameter for ROS accumulation and oxidative stress) of testes in diabetic animals. MDA, a final byproduct of lipids peroxidation by ROS, is an excellent index of ROS accumulation in the tissues. Based on previous studies, occurrence of oxidative stress in testicular tissue affects testicular function and spermatogenesis (25). Cell apoptosis and death due to cellular macromolecules damage is the main detrimental effects of ROS accumulation and development of oxidative damage, which was observed in testicular tissue of diabetic rats (26). According to previous studies, the pro-oxidant enzymes such as NADPH-oxidase are activated as a main mediator of ROS generation in diabetic tissues as well as testicular tissue (6). On the other hand, decreased the antioxidant capacity of testicular tissue is another reason of ROS accumulation in diabetes (27). Therefore, this imbalance between pro-oxidant enzymes and antioxidant defense system would result in ROS overproduction and development of oxidative stress damage in testicular tissue in diabetic state (7). In this regard, a decrease in CAT activity in the present study confirmed weakening of the antioxidant capacity of testicular tissue in diabetic rats. Catalase is one of the main antioxidant enzymes, which performs its antioxidant function by neutralizing H2O2 and converting this toxic radical to H2O (28). Although other parameters of the antioxidant system (GSH level and activity of SOD enzyme) in the testes of diabetic animals did not change in the present study, decreased or increased as well as no change of the antioxidant parameters have been demonstrated in diabetic condition or other pathological states accompanied with tissue oxidative stress (29).
According to the results of the current study, fullerene C60 significantly decreases the oxidative stress indicator, MDA levels, in the testes of diabetic rats. These types of nanomaterials have a potent antioxidant activity (23, 24) and are capable to powerfully remove the different toxic radicals in biological milieus (23). According to a large number of studies, fullerene C60 can scavenge large numbers of free radicals in different tissues (9, 30, 31). These nanoparticles are able to pass all cell membranes and localize between membrane lipids in intracellular organelles, particularly in mitochondria (31). Also, fullerene C60 nanoparticles have been reported to enhance the antioxidant capacity of the cells and tissues (16, 32). According to our results, treatment with fullerene C60 in diabetic rats potentiated the antioxidant capacity of the testicular tissue in diabetic rats through increasing CAT activity. Also, fullerene altered the SOD activity of testicular tissue in normal rats. It has been reported that fullerene C60 behaves as the SOD and CAT mimetics in an in vitro experiment (31, 33). Improving the antioxidant capacity of tissues and increased CAT and SOD activities by fullerene C60 has been reported in diabetic conditions and other pathological states (15, 16, 32). However, according to our results, GSH level and activity of SOD did not show a considerable change in the testicular tissues of fullerene C60-treated diabetic rats. Although several studies have reported the altered SOD activity or GSH content by fullerene C60 (16), others have shown that fullerene C60 has no effects on tissue content or activity of these oxidative parameters (34). It is suggested that the type of pathological states as well as fullerene C60 dosage may be the causes of these inconsistent results. Fullerene C60 administration also improved the weight of testes in diabetic rats. It is suggested that fullerene C60 may ameliorate the weight index of testes through inhibiting the degenerative effects of ROS on testicular tissue of diabetic rats. In this regard, the capability of C60 for scavenging the large numbers of free radicals was proposed as the possible mechanism for fullerene C60-tissue protection.
Beside the beneficial and cytoprotective effects of fullerene nanoparticles, the possible harmful and cytotoxic effects of these types of nanoparticles must be considered. According to findings of the previous studies, these nanoparticles are low toxic substances and no valuable cytotoxic or harmful effects have been reported by administration of fullerene C60 nanoparticles and their derivatives. For example Takahashi et al., reported that the toxicity of oral administration of fullerene C60 is relatively low and no marked changes was detected or observed in the different tissues after long-term administration of fullerene C60 in rats (18). However, Sumi et al., demonstrated that using sublethal concentrations of fullerene C60 nanoparticle in freshwater fish noticeably decreased the antioxidant enzymes activities, and increased h ydrogen peroxide production as well as lipid peroxidation in the gonads (35). Although in the current study we determined the fullerene C60 effect on the antioxidant parameters in the testes of diabetic rats, the hormonal changes, trophic factors, inflammatory and apoptotic signals as well as other parameters related to the reproductive and testicular dysfunction in diabetic condition were not determined. Then, more research is needed to elucidate the effects of fullerene C60 nanoparticle on mentioned parameters in diabetic condition.
Conclusion
It is proposed that fullerene C60 nanoparticle might protect testicular tissue against ROS-induced tissue damage in diabetic states. These protective effects of fullerene C60 may be due to enhancing the antioxidant capacity of the testicular tissue in diabetic animals. Hence, it is suggested that administering fullerene C60 nanomaterials can be useful for improving spermatogenesis and male reproduction system against oxidative stress damages in DM.
Data availability
The raw data supporting the findings of this study are available in case of any request.
Acknowledgements
The authors are thankful to the Vice Chancellor for Research of Baqiyatallah University of Medical Sciences, Tehran, Iran. “IR.BMSU.REC.1397.029” was the Ethical Code for the current study.
Ethical considerations
Ethical issues (Including plagiarism, informed consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc.) have been completely observed by the authors.
Funding and support
This research resulted from an independent research without receiving any financial support.
Conflicts of Interest
Authors declared no conflict of interests.
Received: 2020/04/8 | Accepted: 2020/09/10 | Published: 2020/12/4
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |



