BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://journal.zums.ac.ir/article-1-6287-en.html


 , Zivar Nejad Ebrahimi1
, Zivar Nejad Ebrahimi1 

 , Neda Mahami1
, Neda Mahami1 

 , Mohammad Alizadeh1
, Mohammad Alizadeh1 

 , Zahra Rasooli1
, Zahra Rasooli1 

 , Mina Hemmati *2
, Mina Hemmati *2 


2- Dept. of Biochemistry, School of Medicine, Zanjan University of Medical Sciences, Zanjan, Iran ,
✅ This review study was conducted to evaluate the pharmacological effects of the bisphenol and its signaling pathways especially in the adipose tissue. Studies have shown that the disruption in the level of the adipocytokines can be effective in the formation and progression of the chronic diseases such as cancers.
Many synthetic chemicals are marketed aimed at improving the living standards without considering the negative consequences on human health (1). Bisphenol A (BPA) (4, 4'_dihidroxy_2, 2 di phenyl propane), as a synthesized chemical (material) is formed by the reaction of two phenol groups with an acetone molecule (2, 3). BPA is an estrogen monomer with a molecular weight of 228.29 g / mol and is known by the chemical formula of C15H16O2. BPA is a white crystalline substance with a mild phenolic odor and a melting temperature of 153-159 ˚C (4, 5) which is used as a primary substance for the production of the polycarbonate plastics, epoxy resins, and polyester. Polycarbonates are used in making water bottles, baby food bottles, toys, heat plates, home appliances, sanitary ware, and medical equipment (5). BPA is also utilized in the production of various plastics, such as polyvinyl chloride, which is used in the manufacturing of medical pipes, water pipes, and as a lining in the dentistry (5). Exposure to BPA is mainly through the hydrolysis of plastic containers containing the polycarbonate and epoxy resins. BPA monomers obtained from BPA-produced polymers can be combined with the foods and water; and the consumption of these contaminated foods is the first source of human exposure to BPA (6). Absorption of BPA by the human has been estimated by 10 µg / kg / day (7). During 1995 - 2014, BPA production in the United States and Europe has increased by 37 and 84%, respectively, and this process is expected to increasingly continue (8). Given the human contact with BPA and its harmful effects on the human health, the Environmental Protection Agency (EPA) has introduced BPA as a destructive chemical against the humans' endocrine system (9).
1.Pharmacokinetic of the BPA
In the United States, BPA has been observed in the urine samples of more than 92% of the people at 6 years of age and older (10, 11). The US EPA has stated the reference dose of 50 µg /kg day for BPA. Meanwhile, the European Food Safety Authority (EFSA) has reduced the safe exposure rate to BPA from 50 to 4 µg /kg day (11). Following ingestion, about 90% of BPA passes through the liver and its primary metabolism occurs in the intestine and liver (12,13). Glucuronidated and sulfonated BPAs are among the major secondary metabolites of BPA which are excreted through the urine (Figure 1). BPA mostly acts in the humans' body through binding to the steroid hormone receptors, such as estrogen and androgen receptors to alter the gene expression. The distribution of these receptors varies in different tissues. Therefore, the binding of BPA to these receptors creates a variety of responses on the cell surface. BPA can bind to these receptors both freely and in the conjugated form in the bloodstream and then, exert its physiological functions (13,14).
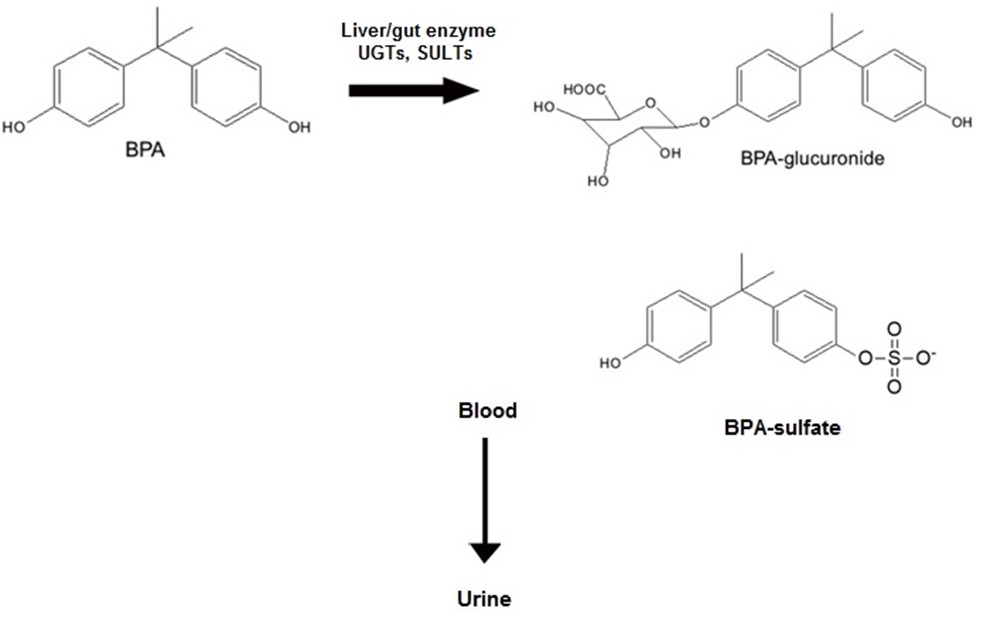
Figure 1. Chemical structure of BPA and its primary human metabolites, BPA-glucuronide. In humans, elimination of BPA is via urine in the form of BPA or BPA conjugates, mostly BPA-glucuronide. The conjugation is done by UDP-glucuronyl transferase (UGT) and sulfotransferase (SULT). Figure was prepared using templates on the servier medical art website (http:// smart.servier.comimage-set-download/).
2. BPA as an Endocrine Disrupting Compound
Endocrine Disrupting Compounds (EDCs) are the compounds altering the function of the hemostatic and hormonal systems. EDCs interfere with the function of the nuclear receptors, estrogenic non-nuclear receptors, non-estrogenic receptors, and neurotransmitter receptors, such as serotonin, dopamine, and norepinephrine receptors, and also the orphan receptors including aryl hydrocarbon receptor as well as the pathways involved in the steroid synthesis or metabolism and other mechanisms conver-ging with the endocrine and reproductive systems (15).
2.1. Estrogen Receptors
BPA can exert its physiological effects through various physiological receptors including the Estrogen Receptors alpha (ER), Estrogen Receptors beta (ERβ), Estrogen-Related Receptors gamma (ERR γ), and Peroxisome Proliferator-Activated Receptor gamma (PPAR γ) (16). Compared to 17β-estradiol (E2), BPA tendency for binding to ERβ is higher than ER (17). BPA acts as an ER agonist and creates the same change as E2, while preventing the formation of the correct conformation of the ligand-binding domain in ERβ and acting as an ERβ antagonist (18, 19). Given the effect of E2 on induction of the cellular proliferation, the effect of BPA has also been investigated in this field. In fact, the balance between signaling of ER and ERβ and the level of these receptors causes the balance between the cell death and proliferation regulated by E2 (20). BPA acts as a proliferative agent by mimicking the E2 function in the presence of ER. While in the presence of ERβ, BPA acts as an antagonist of the E2-ERβ complex indicating that BPA leads to the increase in the cell growth through ER and on the other hand, it reduces the cell death through the antagonistic activity during binding to ERβ (Figure 2). These two functions of BPA in the presence of ERβ and ER can create changes in the cells resulting in the development of cancer (21).
Figure 2. Interaction of BPA with ER/ERresulting in the development of various cancers. In the presence of ER/ERBPA binds to these receptors and induces cell proliferation.
2.2. Androgen Receptor (AR)
BPA acts as an androgen receptor antagonist. Evidence indicates that the antagonistic activity of BPA on the androgen receptor prevents the transmission of the receptor dimer to the nucleus and reduces the expression of the synaptic proteins [Synapsin1 and Postsynaptic Density Protein 95 (PSD95)] in the hippocampus of the male rats resulting in a defect in the spatial memory (22). There is insufficient information on the effect of BPA on the AR transcription activity, and there is not sufficient knowledge about the ability of these compounds to interfere with the androgen-dependent extra-nuclear signals (23). Androgen signaling appears to be less influenced by the intervention of BPA, but BPA can intervene in the treatment of the patients with advanced prostate cancer through mutated ARs (24).
2.3. Thyroid Receptors
BPA has a low affinity for binding to the thyroid receptors (TR) and reduces the self-induction effect of the TR through antagonistic activity and at the same time increases the serum thyroxine (22). BPA inhibits the stimulated transcription activity by Triiodothyronine (T3) through antagonistic activity. Through a Nuclear receptor Co repressor (NCoR), BPA could reduce the expression of the TR gene (5). According to the literature, BPA can influence the expression of the genes involved in the brain development by interfering with the thyroid hormone receptor (25). These effects are mediated by the receptors located in the nucleus, where they act as transcription suppressors in connection with DNA through Silencing Mediator of Retinoic acid and Thyroid hormone receptor (SMRT) and NCoR. The extra-nuclear activity of the TR depends on integrin αvβ3, which is responsible for phosphorylation and activation of the nuclear TR through activating the Mitogen-Activated Protein Kinase (MAP-K) / cellular Sarcoma (c-Src) pathway (26). BPA directly interferes with the β3 / integrin / c-Src / MAPK / TR-β1 pathway at low concentrations, which could affect TR signaling.
2.4. Pregnane X Receptor
Human Steroid and Xenobiotic Receptor / rodent Pregnane X Receptor (SXR / PXR) and Constitutive Androstane Receptor (CAR) are the two important regulators for metabolism of the xenobiotic and steroid hormones (19). These receptors in the liver and intestine mediate the induction of the cytochrome enzymes p450 and transporters such as P-glycoprotein in response to xenobiotic ligands and steroid hormones. SXR / PXR are unusual nuclear receptors, which have extensive ligand specificity and are activated by a lot of EDCs such as BPA. Activation of the SXR / PXR and CAR, and consequently an increase in the expression of their target genes by various components causes an increase in the levels of EDCs and at the same time changes the rate of access to estrogens and androgens (27). In fact, BPA acts as a strong agonist for hPXR. Combination of the effect of BPA and other EDCs results in the synergistic effects on the hPXR function (28).
2.5. Aryl Hydrocarbon Receptor
Aryl hydrocarbon receptor binds to several chemicals and indirectly influences the metabolism of xenobiotics as well as the synthesis and metabolism of steroids. Aryl hydrocarbon receptors are the transcription factors mediating the effects of various chemicals most importantly, Tetrachlorodibenzodioxin. After binding the ligand to the AHR in the cytoplasmic segment, it enters the nucleus by the corresponding transmitter (ARNT) which binds to the responsive elements in DNA and leads to the transcription of the genes involved in the xenobiotic metabolism including CYPs. The AHR repressor must be bound to ARNT to disable this process or the messaging path. BPA disrupts the AHR expression and its activity in the embryo via AHR upstream transcriptional regulation (29). BPA results in the changes in the primary adipogenesis along with hypertrophic modification of the adipocytes andoverexpression of the lipogenic genes such as PPAR γ, Sterol Receptor Element-Binding Protein 1C (SREBP-1C), Lipoprotein Lipase (LPL), and Fatty Acid Synthetase (FAS) (30). BPA is able to increase the expression of the PPAR γ -related-mRNA in the 3T3-L1 pri- adipocytes (31). The cAMP Response Element-Binding protein (CREB) also plays an important role in the differentiation of the adipocytes, and the effects of BPA on the differentiation of the adipocytes are likely to occur through the rapid activation of CREB. Exposure to low BPA concentrations for a long time influences the adipogenic differentiation program by increasing the peri-adipocyte growth and altering the genes regulating the adipogenesis (25). ERRγ is present in the adipose tissue. Evidence indicates that ERRγ with its two isoforms (ERRα / β) plays an important role in controlling the energy homeostasis and biogenesis and the natural function of the mitochondria. Studies have shown that BPA exerts its effects on the body weight regulation through ERRγ (30). BPA binds strongly to the human ERR, and it also binds to ERRγ at low concentrations while maintaining the ERR γ activity in the presence of 4-hydroxydebrisoquine (4-OHD). The effects of BPA at low concentrations on the energy homeostasis are likely mediated by these nuclear receptors (19). BPA acts in the body mainly through the estrogen receptor. This receptor is expressed in various tissues of the body. BPA can cause the disruptions in the tissues of these estrogen receptors by binding to these receptors (32).
3. Expression of the ERs in the Adipose Tissue
Hormones are the main regulators of the adipose tissue function and play an important role in the evolution and development of the adipocytes. A wide range of growth hormones and factors, such as thyroid hormone, glucoco-rticoids, catecholamines, insulin, glucagon, and insulin-like growth factors modify the activity and development of the adipose tissue. Estrogens, especially E2 play an important regulatory role in the metabolism and distri-bution of the adipose tissue (33). The sex steroids contri-bute to the function of the white adipose tissue through controlling the lipolysis, lipogenesis, adipocyte differe-ntiation, insulin sensitivity, and production and secretion of the adipokines (34). In adults, increased estrogen plasma concentration is associated with the decreased food intake and body weight, and estrogen deficiency is accompanied by the initiation of the menopause in overweight women (35). Studies on the humans and laboratory animals have suggested that estrogen is the most important regulator of the white adipose tissue in women(36). Estrogens exert their effects on the adipose tissue through the steroid receptors (37). ER was reported as the first receptor for estrogen in 1973. The second steroid receptor, ERβ was identified in 1996. Studies on the laboratory animals such as adult rats have shown that the endogenous E2 is the most abundant and predominant estrogen, and exerts its inhibitory effect on the number of adipocytes and lipogenic cells through the ER receptor (35). Both ER/β receptors are expressed at the mRNA and protein levels in the human adipose tissue with ER predominance. There is no regional or gender difference in the adipose tissue for ER expre-ssion, while ERβ expression is higher in women than men (38). Different roles have been attributed to ER and ERβ regarding exerting the estrogen effects on the adipose tissue. Obesity has been shown to increase in therats without ER, while normal adipose tissue mass was observed in the rats without ERβ (39). In the studies on the 3T3-L1 cell line, ER receptors have also been observed in the membrane and cell nucleus (40). Estrogens can influence the adipose tissue activity in several ways:
1. A genomic response characterized by the changes in the gene transcription that occurs from several hours to several days.
2. Non-genomic signal events that occur from several seconds to several minutes after cell stimulation (21).
With regard to the presence of the ERs in the adipose tissue and their activities in the adipose tissue, BPA can cause the disruptions in the adipose tissue by the ERs. These disruptions include the changes in the normal secretions of adipocytokines from this tissue (41).
4. Disruption in the Secretions of the Adipose Tissue Leading to the Development of Cancer
The adipose tissue is a dynamic tissue involved in regulation of the glucose and lipid metabolism, energy homeostasis, and inflammation. In addition, the adipose tissue is an active and complex endocrine organ playing a role in the metabolism of sex steroids and the synthesis and secretion of the active adipokines (42). In addition to storing fat, the white adipose tissue is an active endocrine gland that secretes more than 50 cytokines and other molecules generally called adipocytokines including adiponectin (APN), leptin, Tumor Necrosis Factor α (TNFα), and Interleukin 6 (IL-6) (43, 44). There is a special relationship between the adipose tissue and different types of cancer including colorectal cancer, kidney cancer, postmenopausal breast cancer, esophageal cancer, etc. (45). The balance between the number of adipocytes and cancer cells is created by cytokines including IL-6, TNFα, and adipokines, such as APN and other proteins produced from the adipose tissue to control invasion and proliferation of the cancer cells (46). Recent studies have confirmed the effects of the adipocytokines in cancer (47). According to the recent studies, BPA can cause changes in the secretions of the adipose tissue through different cellular pathways (1). The following investigates the effects of BPA on adipocytokines and the role of these changes in cancer in detail.
5. The Effects of BPA on Cytokine Secretion
Numerous reports have shown that BPA influences the levels of important hormones associated with metabolism, such as APN and leptin which are among adipocytokines (48). EDCs can disrupt the signaling by the regulatory hormones including APN and leptin (32). Regarding the presence of BPA around the world as well as the constant exposure of humans to the it, inhibition of APN secretion, and increased levels of the IL-6, TNFα, and leptin at nanomolar concentrations may be the important factors in the endocrine disruption and homeostasis of metabolism (18).
5.1. The Effects of BPA on the APN Secretion from the Adipose Tissue
APN is the most abundant adipocytokine secreted by the adipocyte cells of the adipose tissue. APN, as an adipokine is responsible for energy storage in the form of TG, and has anti-apoptotic and anti-inflammatory activities (49). Clinical studies have shown that the APN level plays an important role in the protection against the carcinogenic factors. Some cancer cells influence the APN receptors and their downstream signaling pathways to prevent the apoptosis (50). APN can prevent the development of the cancers such as breast cancer, lung cancer, thyroid cancer, and colorectal cancer by regulating the signaling pathways, apoptosis, and the cell cycle (51). Atrial Natriuretic Peptide (ANP) is a mediator between the AMP-activated Protein Kinase (AMPK), Janus Kinase (JAK) / Signal Transducer and Activator of Transcription 3 (STAT3) ,and MAPK signaling pathways (51, 52). ANP causes the activation of apoptosis and inhibition of the cell proliferation through the AMPK signaling pathways (52). Studies conducted on humans and animals have shown that estrogen reduces APN secretion. Results of a study on the rats without the aromatase enzyme showed that the APN levels were reduced by E2 consumption (53). EDCs act directly on the steroid hormone receptors or indirectly through non-steroidal receptors. Like E2, BPA activates the ERɑ-dependent signaling pathway leading to the MAPK / Extracellular-Regulated Kinase (ERK), and Protein Kinase B (AKT) phosphorylation (54). Low BPA concentrations inhibit the APN secretion in the human adipose cells in -vitro. It has been observed that BPA accumulates in the adipose tissue. In addition, the ERRβ receptor, as a nuclear receptor has a high tendency for binding to BPA (55).
BPA regulates the expression and phosphorylation of AKT acting on the downstream of the Phosphoinositide 3-Kinase (PI3K), and the PI3K signaling pathway is accompanied by the production and secretion of APN. The PI3K-AKT pathway is activated when the insulin hormone binds to its receptor at the cell level that plays a key role in regulating the gene expression and APN secretion. In fact, BPA inhibits the expression and phosphorylation of AKT, and APN levels have been reported to be significantly lower in the cell cultures treated with BPA. BPA does not act through the nuclear ER pathway in the 3T3-L1 cells, but it also binds to the estrogen-related receptor in the human (56). In fact, PI3K, MAPK, and ER have been reported as the main components of the signal transmitter for E2 or as foreign estrogens in the regulation of the genes in the 3T3-L1 cell line (40). Both BPA and E2 inhibit the APN secretion, but they do not act through a single mechanism (32). APN synthesis is stimulated by PPAR, insulin, and Insulin-like Growth Factor-1 (IGF-1) agonists. Increased expr-ession of APN by PPAR is highly regarded, because being an antagonist for PPAR is among the mechanisms through which BPA can suppress the APN secretion.The APN secretion is controlled at three levels: biosynthesis, assembly, and release (57).The APN synthesis is stimulated by PPAR, insulin, and IGF-1 agonists and is inhibited by TNF- and catecholamines (18). Increase in the APN expression is important because the mechanism by which BPA can suppress the APN secretion is performed by the PPAR antagonist. In fact, conjugated compounds with BPA are potential antagonists for PPAR activities in the adipocytes (58). In addition, BPA can inhibit the Protein Disulfide Isomerase (PDI) enzyme playing an important role in the formation of the disulfide bonds in APN, which is considered as a mechanism involved in the reduction of APN by BPA (59).
5.1.1. Decreased Level of APN and Its Association with Various Cancers
Endometrial Cancer (EC)
There is an inverse relationship between the serum level of APN and the risk of endometrial cancer (60). APN reduces the viability of the EC cells and decreases their number through inhibiting the proliferation and inducing the apoptosis (50). Previous studies have shown an inverse relationship between the serum APN level and the risk of EC in the women under 65 years of age (51, 61, 62).
Hepatic Cancer (HC)
The risk of liver cancer is directly associated with the serum level of APN (50, 63). APN responds to liver cancer by preventing the cell proliferation and pro –apoptotic factors. The expression of Cyclin D1 and Proliferating Cell Nuclear Antigen (PCNA) decreases and instead, the expression of Caspase -3 increases in the liver cancer cells. APN influences the hepatocellular cancer cells through AMPK, c-Jun N-terminal Kinase (JNK), and mammalian Target of Rapamycin (mTOR). APN causes the JNK phosphorylation in the HepG2 and Huh7 cells and increases the AMPK activity through phosphorylation (63).
Prostate Cancer (PC)
Prostate cancer is one of the most common cancers in men metastasizing to other organs. Clipping the androgen pathway is among the therapeutic methods for treatment of PC; however, this therapeutic method does not respond in some cases. APN level is lower than the normal in the patients with PC and is inversely related to the disease progression (50, 61, 63-65). The mRNA expression of AdipoR1 / R2 receptors is also reduced in the PC cells. Serum APN level and profile amount of its receptors can predict the risk of PC (51, 52, 65). APN influences the pathogenesis of PC through the JNK and STAT3 pathways. ANP causes the activation of the JNK, AMPK, and PI3 kinase / AKT pathways in the PC cells. The AMPK pathway inhibits the growth and proliferation of the PC cells through 5-aminoimidazole -4-carboxamid ribose (AICAR) (51, 63, 66).
Gastric Cancer (GC)
Low serum level of APN is directly associated with an increased risk of gastric cancer. Increased expression of the AdipoR1 receptor suppresses the growth of the GC cells (50, 51). Serum level of APN is lower than the normal range in the patients with esophageal cancer. Esophageal cancer is among the malignant cancers metastasizing to the gastrointestinal tract and in most cases, the patients die within 5 years (50, 67). According to literature, low serum level of APN also increase the risk of pancreatic cancer (50).
Colorectal Cancer (CRC)
Numerous studies have proved an inverse relationship between the serum level of APN and the risk of colorectal cancer (45, 46, 50, 63, 66, 68, 69). CRC is among the most common cancers associated with obesity. Previous studies have shown that low serum level of APN is strongly associated with an increased risk of CRC (70). AdipoR1 / R2 are expressed in the CRC cells and also involve the lymph nodes. In the low stages of the disease, the rate of AdipoR1 expression is associated with the tumor size (46, 63, 64). High serum level of APN reduces the risk of CRC by 60% (50, 71). Inhibition of the Cyclin-Dependent Kinase (CDK) inhibitors, P21 / P27 through AdipoR1 / R2 increases the cell cycle suppression. Therefore, APN can control the proliferation of the cancer cells by activating several tumor suppressor genes (68).
Thyroid Cancer (TC)
Thyroid cancer is the second most common cancer in young women. APN receptors are expressed both in the healthy thyroid and TC cells (46). Plasma APN level is lower in patients with TC than the healthy individuals (60).
Renal Cell Cancer (RCC)
Low serum level of APN not only increases the risk of kidney cancer but also causes metastasis. The expression of AdipoR1 / R2 receptors is lower in the kidney tissues than the other tissues resulting in a decrease in the pro-tective potential of APN in kidneys (50, 60, 64, 70, 72).
5.2. The Effects of BPA on the Leptin Secretion from the Adipose Tissue
Leptin, as a 16 -kDa peptide hormone contains 167 amino acids. The three-dimensional structure of leptin consists of a motif containing four alpha helixes, which is very similar to the structure of the (IL-6) family (73) .In terms of the structure, leptin is a typical neuropeptide with anorexic function. It is one of the important molecules involved in the development of obesity, which is also called the "satiety hormone" because it plays a key role in absorbing the nutrients and controlling the energy consumption. It is derived from the Greek word "leptos", which means the slender and thin (74). Vokberg et al., showed that the exposure to BPA leads to weight gain and accumulation of the adipose tissue mass, it also creates some changes in the leptin level (75). Increased serum level of leptin is associated with several cancers including CRC, PC ,and Breast Cancer (BC) (76, 77). Leptin increases the proliferation, migration, and invasion of the cancer cells (69, 78) and is able to induce the vascu-larization, increase the level of the Vascular Endothelial Growth Factor (VEGF), and suppress the secretion of the anti-inflammatory cytokines (79). Binding the leptin to its receptor activates several signaling pathways in the form of a cascade. So that, first, tyrosine kinase is activated, and influences the cytokine receptor and binds it to the transcription activators of STAT. STAT migrates toward the nucleus and induces the expression of the genes, such as down-regulators (including the cytokine signaling inhibitors) like JAK2-STAT3, MAPK, and PI3K / AKT (80). Studies have shown that the leptin levels in the bloodstream are higher in women than men. An increase in the mRNA of Ob (human Obesity) gene in the presence of E2 reflects the direct effect of estrogen on the transcription of Ob gene. Evidence indicates that there are several responsive elements to the Specificity Protein 1(SP1) in Ob gene promoter in humans. Thus, ER-SP1 relationship repre-sents the transcription by estrogen and without the need for direct binding of ER to the DNA. In -vitro studies have shown that the treatment of adipocyte cells of the rats with estrogen increases the leptin secretion (81). Specific activities of leptin in target tissues are conducted by its interaction with specific receptors, leading to the secondary activation of the JAK / STAT, MAPK / ERK, and PI3K / AKT pathways (82).
Previous studies have shown that BPA and leptin can initiate similar intracellular messaging pathways that induce cell proliferation. It has also been shown that BPA increases the leptin receptor expression and, like leptin, induces proliferation by activating the STAT3, ERK1 / 2, and AKT messaging pathways. Similar to one of the xenoestrogens, BPA increases the expression of the leptin receptor gene in the ovarian cancer cells and activates the intracellular signaling pathways (83, 84). There is normal overexpression in the amount of leptin and its receptor in the advanced stages of the BC (83). Leptin can bind to all its four receptor forms (ob-Rd, ob-Rc, ob-Ra, ob-Rf) and activate the JAK pathways, and phosphorylate the Insulin Receptor Substrates (IRS), and initiate the AKT / PI3K phosphorylation. Increased expression of the leptin receptor in the cancer cells is associated with the decre-ased survival (69). Leptin inactivates various signaling pathways including the AKT / PI3K, JAK / STAT, and MAPK / ERK pathways (76, 83-85).The AKT / PI3K pathway plays an important role in regulating the cell growth and preventing the cell proliferation through apoptosis (76). Estrogen is among the compounds that can influence this pathway, which prevents the apoptosis by activating the PI3K / AKT and MEK / ERK pathways, as a common pathway between leptin and estrogen (78). Leptin increases the D1 and c-Myc Cyclin expression, and also increases the rate of cell cycle progression by reducing G1. Cyclin D1 is overexpressed in the BC cells. Cyclin D1 plays an essential and important role in regulating and advancing the cell cycle from stage G1 to S (78). Leptin reduces apoptosis through upregulation of the growth stimulants such as the Cyclin D1 (to intensify the cell proliferation) and downregulation of the tumor suppressors such as P53 and P21 (78). Leptin produced by the adipose tissue adjacent to the tumor causes a local increase in the leptin level and stimulates the tumor growth. In other words, the secretions of the adipose tissue around the tumor into the intercellular space play an important role in the local growth of the tumor (69). The effects of BPA on adipokine secretion and related signaling pathways are summarized in Figure 3.

Figure 3. Schematic representation of estrogenic activity of BPA in adipocytes through genomic and non-genomic pathways, as described in the text. BPA mediates transactivation of OB gene. BPA also inhibits APN expression through inhibition of expression and phosphorylation of AKT. Figure was prepared using templates on the servier medical art website (http:// smart.servier.comimage-set-download/).
5.2.1. Increased Level of Leptin and Its Association with Various Cancers
Colorectal Cancer (CRC)
Leptin is usually released within the cycle of 2 - 3 hours after a meal, and serum leptin is directly related to the fat reserves; that is, it increases under the obesity conditions and decreases following the weight loss. It has been observed that if the number and size of the fat cells increase, the LEP gene is activated with the synthesis of leptin, which is then secreted into the bloodstream. CRC occurs more in the obese people than the healthy people. Numerous reports have shown that leptin plays an important role in the progression and pathogenesis of CRC. Leptin receptor expression has been observed in the cytoplasm and the cell membrane of many tumor cells including the CRC cells. Thus, it can be said that, the leptin receptors are expressed in 77-95.5% of CRC cases (84). Evidence indicates that obesity has a negative effect on the cancer treatment and leads to the development of drug- resistance, especially through leptin and its receptor. Leptin adipokine, has been suggested to strengthen the tumor and therapeutic resistance in many cancers. Cytokine leptin increases the pre-inflammatory and immune secretion of the cancer cells (86). Leptin also forms a tumor microenvironment to strengthen the angiogenesis and metastasis. In addition, leptin is associated with the expansion of the cancerous stem cells population, and the expression of the leptin receptor is essential for maintaining the properties of its cancerous stem cells so that, leptin production increases the soluble Intercellular Adhesion Molecule-1 (sICAM-1) in the cancerous cells. ICAM-1 expression has not been observed in the lung and breast cancerous cells, and this effect is regulated by the leptin receptor (ObR). In addition, leptin prescription causes an increase in the exp-ression of ERK, JAK1 / 2 STAT3, and Focal Adhesion Kinase (FAK) genes and also leads to the incidence of ICAM-1 expression (86). Numerous studies have confirmed that the Leptin Receptor (LEPR) signaling can cause the adhesions, angiogenesis, migration, and survival of the cancerous cells. A significant increase in the LEPR expression in patients with metastatic forms of CRC indicates that the LEPR activity is a valuable diagnostic indicator of the metastatic potential of CRC (74).
Breast Cancer (BC)
Leptin is the most important mediator of obesity and breast cancer, leading to tumorigenesis, development, growth, and metastasis. Molecular mechanisms assoc-iated with obesity and BC are complex mechanisms, but four mechanisms can explain this relationship including the expression of the leptin receptor, chronic inflame-mation of the adipose tissue, sex hormone replacement (IGF-I), and insulin signaling. Leptin increases the risk of BC by cross-reacting with other signaling molecules including ERα, growth and Notch factors, and inflame-matory factors. Cross signaling between leptin and IGF-I causes invasion of BC through the reaction of the Epidermal Growth Factor Receptor (EGFR). Mutual signaling between the leptin-Notch and interleukin-1 (NILCO) causes the development of the cancer. Leptin –NILCO axis is a mediator of activation of the stem cells. Signaling of the cancer stem cells strengthens the expression of the leptin receptors by activating the STAT3 pathway and increases the expression of the stem cells' markers such as the octamer-binding transcription factor 4 (OCT4) and (sex determining region Y)-box 2 (SOX2) (87).
Ovarian Cancer
Leptin and its receptor are overexpressed in the epithelial ovarian cancer, which is significantly associated with the progress-free survival. Increased expression of the leptin receptor gene and protein in the ovarian cancer cells occurs at nanomolar concentrations. Previous studies have shown that leptin and BPA inhibit the expression of the Caspase -3 gene in the OVCAR-3, as the ovarian carcinoma cell line. However, there is no information on the interaction between BPA and leptin in inhibiting the apoptosis in cancer. Activation of the JAK / STAT, MAPK / ERK1 / 2 ,and PI3K / Akt signaling pathways has been investigated previously, and it has been hypo-thesized that two distinct combinations of leptin and BPA (obesity-related) may regulate the apoptosis through activation of the intracellular targets in the OvCAR-3 ,as the ovarian cancer cells. It has been shown that BPA and leptin interact to inhibit the gene and protein Caspase -3 through different intracellular signaling pathways so that leptin works through STAT-3, while BPA works through ERK1 / 2 (83).
Thyroid Cancer (TC)
Leptin may influence the migration of the thyroid cells. Also, adipokines, such as APN, leptin, and hepatocyte growth factors are able to regulate the proliferation and invasion of the cancerous cells and may play a role in the development of TC. Leptin serum level increases in the patients with Papillary Thyroid Cancer (PTC). Decreased APN level known as the angiogenesis inhibitor is accompanied by the progression of TC under obesity con-ditions, and changes in the thyroid function and potential mediators, such as cytokines, insulin, leptin, and APNs may be related to an inflammatory condition (79). Numerous clinical studies have shown that the lymphatic invasion is more frequently observed in the cancers with high leptin expression in different types of tumor. Grow-ing evidence shows that leptin increases the invasion and proliferation in the breast, ovary, lung, bile, and pancreatic cancers and other cancers. Previous studies have demonstrated that leptin is expressed in the cell categories prone to the migration, and exogenous leptin also increases the ability of the glioma cells to migrate. In addition, it has been reported that leptin plays a role in the activity of the cancerous fibroblasts and the expression of the matrix metalloproteinases greatly influencing the metastatic behavior of the cancerous cells. In addition, there is also evidence showing that leptin increases the transmission of the epithelial cells through various signaling mechanisms leading to the tumor invasion and metastasis (86).
5.3. The Effects of BPA on the IL-6 Secretion from the Adipose Tissue
IL-6, as a 184-aa cytokine with a molecular weight of about 25 KDa and pleiotropic function has both anti-inflammatory and pre-inflammatory properties in the immune and non-immune cells (88, 89). IL-6 is synthesized in the immune cells and the peri-adipocytes and adipocytes. IL-6 production is mediated through stimulation by insulin through the cyclic Guanosine Monophosphate (cGMP) / Protein Kinase G (PKG) / CREB pathway, also requiring MAPK (86). Evidence indicates that nanomolar concentrations of BPA may induce a similar response of inflammation in the human adipose tissue. Exposure to BPA causes the secretion of the inflammatory factor of IL6. The activation of normal inflammatory pathways is another finding associated with the disruptions caused by BPA. In fact, the JNK, JAK / STAT, and (nuclear factor kappa light chain enhancer of activated B cells) NF-KB pathways have been shown to be activated in the adipose tissue treated with BPA (86). It is assumed that BPA can stimulate the Toll-Like Receptors (TLRs) and leads to the proper regulation of pre-inflammatory factors such as IL-6 through downstream signaling of JNK and NF-KB (86).BPA may activate the intracellular signaling pathways through TLR and ER ,or GPRC30, leading to the upregulation of IL6, which in turn can stimulate and activate the common inflammatory pathways such as JNK and JAK / STAT pathways (86).After binding of IL-6 to its receptor (IL-6R), it is bound to another receptor called glycoprotein 130 (gp130) and then, signaling is started (86. Dimer form of gp130 activates the tyrosine kinase of JAK1leading to the activation of the other signaling pathways including the STAT1, PI3K, MAPK, and STAT3 pathways (88-90). STAT3 acts as an oncogen and is the linker between inflammation and cancer. Activation of SATA3 by IL-6 increases the expression of Cyclins B1, D2, D1 ,and C-Myc and inhibits the expression of the CDKs, as a result of which the cell enters the cell cycle. It also increases the expression of a number of survival proteins including the (B-cell lymphoma 2) Bcl-2, (myeloid leukemia cell differentiation protein) Mcl-1, Survivin , and (B-cell lymphoma-extra-large) Bcl-xl. Increased expression of these proteins and continuation of the STAT3 activity lead to the drug resistance in cancer patients. STAT3 activity induces the expression of the Hypoxia-Inducible Factor α (HIFα) dependent on the VEGF downstream protein of this pathway (88, 91). Activating the JAK-STAT3 signaling pathway leads to an increase in the expression of the matrix metalloproteinase (MMP7, MMP2, and MMP9). MMP9 plays an important role in the destruction of the extracellular matrix and causes invasion of the tumor cells (91). Cancer is also an inflammatory disease, and the expression of the cytokines plays a major role in the incidence of cancer (88, 92). Increased expression of IL-6 is associated with the cancers such as colon, lung, prostate, liver, etc (88).
Twenty percent of the cancers are caused by the chronic inflammation. IL-6, as one of the pro- tumorigenic cytokines controls and regulates the proliferation of the cancer cells by regulating the signaling pathways of growth factors including EGF and HGF family members (91). TNFα increases the IL-6 expression during the inflammatory response (88). Increased expression of IL-6 in several organs such as the brain, lungs,the liver, and the bone marrow causes absorption and metastasis of the circulating tumor cells in these organs. Activation of NF-κB in the Kupffer cells stimulates the production of IL-6causing the metastasis of the lung cancerous cells to the liver (91).
5.3.1. Increased Level of IL-6 and Its Association with Various Cancers
Colorectal Cancer (CRC)
CRC is accompanied by an increase in the IL-6 secretion, which also increases with the progression of the cancer, but it is not considered as a prognostic factor of the cancer. IL-6 increases the proliferation and growth of the CRC cells. Proliferation and survival of the cancer cells induced by IL-6 depend on the STAT3 activity in anthrocytes (91, 93). In this type of cancer, IL-6 stim-ulates the proliferation of the epithelial cells through NF-κB and STAT3 cascading pathways (88).
Gastric Cancer (GC)
STAT3 activity is associated with the reduced survival (viability) in the patients with gastric cancer. Studies have shown that the IL-6 activity and function are associated with the enhanced initiating factors of GC. IL-6 is the ligand -binding receptor of gp130 and strongly and effectively upregulates gp130 in the gastric tumors (91).
Liver Cancer
HCC, as a common liver cancer is often caused by the inflammation due to the chronic infection with the hepatitis C and B viruses. IL-6 is strongly associated with HCC, and the higher the serum level of IL-6, the larger the size of the tumor. IL-6R and gp130 are highly expressed in the hepatocytes (91).
Pancreatic Cancer
Increased STAT3 activity has been proven in patients with pancreatic cancer. Serum level of IL-6 is higher in patients with pancreatic cancer than the normal individuals. STAT3-activated MMP7 is required for tum-or progression but is dependent on other STAT3 targets for tumor initiation (91).
Prostate Cancer
IL-6 and its receptor are highly expressed in PC. STAT3 activity is inversely related to the progression and metastasis of PC (91).
Kidney and Bladder Cancers
IL-6 level is related to the tumor size, stage, and tumor progression, and it is also a factor in metastasis of the kidney and bladder tumors (91). The autocrine effect of IL-6 in kidney cancer is associated with P53 (94).
5.4. The Effects of BPA on the TNF-α Secretion from the Adipose Tissue
TNFα is a potent cytokine with an essential role in the intrinsic immunity and also a transmembrane protein with 26KDa molecular weight that is expressed in a wide range of cells, in addition to the macrophages including the adipose tissue as well as the breast, colon, and pancreatic cancer cells. TNFα has two types of receptors (92, 95-97):
1) R1 (also called as P55)
2) R2 (also called as P75)
R1 is expressed in all cells, and R2 is selectively expressed in the endothelial and immune system cells. TNFα has the same tendency for binding to both receptors. TNFα is essential for the proliferation and pro-per functioning of the T cells, B cells, macrophages, and dendritic cells (92, 95, 96).
Studies on the effect of BPA on the TNFα secretion from the adipose tissue have proven that BPA increases the expression and secretion of TNFα from the adipose tissue cells through binding to the classic ERs present on the surface of the adipose tissue (30, 98). Lack of TNFα regulation is associated with the extensive changes, such as the development of cancer. TNFα is associated with all stages of cancer, such as tumorigenesis, cellular changes, survival, proliferation, invasion, angiogenesis, and metastasis (95). Most chronic inflammatory diseases increase the risk of cancer, and it has also been proven that most cancers develop in the chronic inflammatory sites. TNFα is not usually detectable in the plasma of healthy individuals, for example, in patients with PC, TNFα and IL-6 levels are associated with the cancer progression (96). Tumor cells that have high growth and proliferation and migrate rapidly induce the TNFα expression, and this cytokine increases the expression of the Vascular Cell Adhesion Molecule-1(VCAM1). As a result, comm-unication and interaction is established between the tumor and endothelial cells resulting in the colonization in the tissues and tumor metastasis to other tissues (99, 100). TNFα induces the expression of the MMPs and dipeptidyl peptidases and causes the growth, migration, and invasion of ERs in the MCF7 and MDA-MB231 cell lines (101). MMPs are the enzymes that regulate the extracellular matrix and play a role in the angiogenesis, growth, proli-feration, and metastasis of various tumors including the BC cells. Epithelial-mesenchymal transmission is among the other roles of TNFα in the progression of cancer. During this process, epithelial cells lose their phenotypic properties and increase the mesenchymal properties such as loss of the E -cadherin (epithelial marker), induction of vimentin, loss of the cellular adhesion, disruption of the cell adhesion, resistance to apoptosis, and increased mobility and invasion (101).
5.4.1. Increased Level of TNF-α and its Association with Various Cancers
Kidney Cancer
TNFα and TNFR2 are expressed in the renal malignancies. Serum level of TNFα is significantly higher in patients with kidney cancer than the healthy individuals and increases with the progression of the cancer (102). TNFα level is associated with the tumor size in kidney cancer. TNFα exacerbates the EMT (epithelial-mesen-chymal transitional) expression and tumorigenesis in the RCC through suppression of the E -cadherin, regulation of the vimentin expression and MMP9, and increased invasion to other tissues (103).
Breast Cancer (BC)
Breast cancer often develops after a chronic inflammation and in the presence of the immune cells, such as neutrophils, macrophages, TNFα, lymphocyte B, and so on. TNFα is not detectable in the serum of the healthy women, but in the women with BC, the level of cytokines, especially the TNFα is elevated and measurable (103).
Pancreatic Cancer
Pancreatic cancer is one of the fatal cancers and the patients with pancreatic cancer do not survive more than 5 years. Pre-inflammatory cytokines as well as the IL-6, IL-17, and TNFα play an important role in the pancreatic cancer. Pancreatic tumor cells can also produce the TNFα at picograms inducing the expression of the EGFR and Transforming Growth Factor alpha (TGFα), as the mediators for proliferation (93, 96).
Conclusion
BPA is a synthetic chemical that is increasingly found in the human living environment. This compound has a great effect on the signaling of the adipose tissue through the ERs, which can lead to the changes in the level of the adipocytokines including TNF-α, IL-6, leptin, and APN. Imbalance in the secretion of these adipocytokines can lead the cells to be cancerous. Findings of the existing researches showed that BPA increases the secretion of TNF-α, IL-6, and leptin through various signaling pathways and in contrast, it reduces the level of APN. Given the protective role of the APN, targeting pathways increasing the level of this adipokine will be effective in reducing the side effects of BPA, which requires to be considered in environmental investigation in the further researches.
Acknowledgements
None.
Conflicts of Interest
The authors confirm that there are no conflicts of interest.
Received: 2020/11/7 | Accepted: 2021/03/15 | Published: 2022/01/31
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |




