BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://journal.zums.ac.ir/article-1-6365-en.html
2- Social Determinants of Health (SDH) Research Center, Kashan University of Medical Sciences, Kashan, Iran ,
3- Dept. of Medical Surgical Nursing, Faculty of Nursing and Midwifery, Kashan University of Medical Sciences, Kashan, Iran
4- Dept. of Cardiovascular Medicine, Kashan University of Medical Sciences, Kashan, Iran
✅ The mouthwash containing PSO improved chemotherapy-induced mucositis further than the routine mouthwash. Therefore, PSO can be used along with routine medicinal solutions to relieve and prevent oral mucositis symptoms.
Oral mucositis (1) is a painful and disturbing oral inflammation caused by induction of chemotherapy. High-dose myeloablative chemotherapy often causes complications such as erythema, edema, atrophy and oral mucositis (2). Clinically, mucositis occurs shortly after the onset of chemotherapy and peaks within 7-10 days, and sometimes up to two weeks after the treatment (3). Usually, 10%, 40% and 80% of the patients undergoing adjuvant, induction and high-dose chemotherapy experience OM, respectively (4).
Pain in OM can be so excruciating that it may affect food intake, oral care and quality of life of the patients (5). Severe OM may lead to oral mucosal infections and bleeding, a decrease in the chemotherapy dose, refusal to continue treatment, additional costs, feeding through a gastrostomy or intravenous route, reduced quality of life, and life-threatening issues(6).
Several topical antibacterial, anti-inflammatory and antihistaminic agents, topical anesthetics and glucocorticoids have been suggested to relieve OM symptoms (7).
Currently, the medical community and patients are interested in the use of herbal products due to their therapeutic properties, fewer complications, better compliance, lower costs, and better adaptation to the physiological function of the body (8, 9).
Pomegranate (10) has been used to kill parasites, purify blood, decrease blood sugar in diabetes, treat aphthous stomatitis, and gastrointestinal ulcers in Indian and Greek medicine (11). Over the past decades, antioxidant and anticancer properties, cardiovascular effects, antibacterial, antiviral and antifungal properties, effects on skin diseases, oral diseases and dental cavities, bone diseases, and obesity have been reported for pomegranate(12). Flavonoids in pomegranate seed oil (PSO) have anti-inflammatory, antibacterial, and anti-cancer effects (13). Furthermore, anti-inflammatory, antimicrobial, antiviral (14), anti-candida (15), and anti-cancer properties of PSO have been investigated and reported in numerous studies (16, 17).
Antibiotics and sometimes some topical or systemic anti-inflammatory medications are needed to treat OM (18). Taking such medications is associated with multiple side effects, allergies, and drug resistance in patients (19). Therefore, the use of herbal products, such as PSO, can be helpful due to their anti-inflammatory and antimicrobial properties (20).
Given the high prevalence of chemotherapy-induced OM and its consequences, and the positive therapeutic effects of PSO on other diseases, and the lack of studies in this regard, this study aimed to investigate the effects of PSO on improving chemotherapy-induced OM in patients presenting to Shahid Beheshti Hospital in Kashan, Iran.
Study type and participants
This, single-blind, randomized, controlled clinical trial recruited 70 patients with chemotherapy-induced mucositis in the Oncology Ward of Shahid Beheshti Hospital in Kashan, Iran from December 2018 to February 2019. Sample size was calculated 35 cases in each group of the study according to a previous study (21) with the aim of “determining the effect of Punica granatum extract on the treatment of Candida-associated stomatitis” and antifungal effects of Miconazole (90%) and Punica granatum extract (70%), and given a maximum error of 30% for no difference between the intervention and control groups (Noninferiority assumption), 95% confidence, and 80% test power.
The inclusion criteria included undergoing chemotherapy without concomitant radiotherapy, OM (grade 1-3), age over 18 years, complete consciousness, consent to participate in the study, no known cognitive impairment, no known underlying diseases (e.g. renal, hepatic, respiratory diseases and immunodeficiency), no history of allergies including rhinitisand asthma, no history of allergies to herbal products, and no use of systemic antibiotics and antifungals at the beginning of the study. The exclusion criteria were fever, using mouthwashes other than those specified during the study, patients’ decision to withdraw from the study, irregular frequency, amount or time of mouthwash use, and death of the patient.
Sampling
The researcher provided eligible patients with comprehensive explanations about the study, and if they filled the informed consent form, they were assigned into either the intervention (N=35) or the control group (N=35) via block randomization and using a random number table. First, block sizes of 4 were prepared from letters A and B (6 blocks) and numbered from 1 to 6. Then, a number from 1 to 6 was selected randomly using a random number table, and the composition of each block was written in accordance with the selected number. As such, a sequence of letters A and B was made, each representing one of the treatment or placebo groups. Patients were assigned to one of the groups according to the above table on their arrival if their disease was confirmed (Figure 1). The patient's participation in the study was voluntary, and they were free to leave the study at any stage.
The control group received routine mouthwash (1, 3). The intervention group received a mixture of routine mouthwash (half of routine dosage) and PSO (50:50). Ultra-virgin PSO, prepared by the cold press and commercially packaged, was procured from Ganjineh Osareh Tabeeat Isfahan Co. (license number: 104.7533.1335328).
All the patients received individual face-to-face training about oral care procedures and the use of toothbrushes and mouthwashes by the researcher. Both groups were taught to wash their teeth with a soft toothbrush and toothpaste 4 times a day (after each meal and before going to bed) and then keep 15 ml of the mouthwash in their mouth for 3 minutes and gargle and then discard it for 14 consecutive days. Patients were asked not to wash their mouths or eat for an hour after using the mouthwash. In order to ensure the correct use of the mouthwashes, patients or their companions were trained to record the amount and timing of using the mouthwashes in a checklist 4 times a day for 14 consecutive days. Patients were asked to store the bottle of mouthwash in the refrigerator after each use. If patients did not use mouthwashes according to the instructions given, they were excluded and replaced with a new patient.
Ethical considerations
This study was conducted in accordance with the instructions published in the Declaration of Helsinki. Necessary permissions were obtained from the Ethics Committee of Kashan University of Medical Sciences to conduct this study (IR.KAUMS.MEDNT.REC.1397.32) and the study was registered on the website of the Iranian Registry of Clinical Trials (IRCT20100829004655N10).
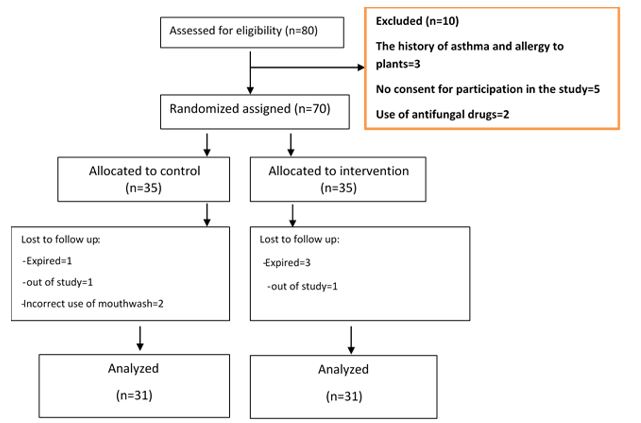
Figure 1. Consort flow diagram of the study
Data collection tools
A demographic information questionnaire and a checklist for assessing the severity of OM were used to collect the data.
The demographic information questionnaire had two parts for personal and medical record information (age, sex, marital status, educational status) and the questions regarding the duration of illness, the duration of chemotherapy, treatment regimen (type, dosage and prescribed medications), and type and duration of the anti-inflammatory regimen. The severity of OM was investigated based on a 5-degree checklist based on the WHO standard protocol (22) (Table 1).
Table 1. World Health Organization (WHO) scale for oral mucositis
| No oral mucositis | Grade 0 |
| Erythema and Soreness | Grade 1 |
| Ulcers, able to eat solids | Grade 2 |
| Ulcers, liquid diet (due to mucositis) | Grade 3 |
| Ulcers, alimentation not possible (due to mucositis) | Grade 4 |
Patients’ oral mucosa was examined and recorded on the 1st, 7th and 14th days using mouth mirrors and tongue depressors by an oncologist and a hematologist. Scores of zero to four were given according to table 1. Validity and reliability of the checklist were assessed through several studies (23, 24). The content validity of the Persian version was approved and its reliability was 0.93(23).
Statistical Analysis
The Kolmogorov-Smirnov test was used to check normal distribution of data, which was approved. Fischer’s exact and Chi-square tests were used to compare the two groups in terms of categorical data. Data were analyzed based on the intention-to-treat approach. First, the means and standard deviations of severity index of oral mucositis were calculated then independent t-test was used to compare the rates of severity of OM between the two groups. Finally, repeated measures ANOVA was used to analyze the effect of time and treatment group on changes in the severity of OM. Data were analyzed in SPSS software-16, and the significance level was set as 0.05.
The mean ages of the participants in the intervention and control groups were 57.48±14.742 and 58.81±14.134 years, respectively. The two groups were matched for clinical and demographic characteristics (P>0.05, Table 2). The severity rates of OM decreased from 1.97 on the 1st day to 0.52 on the 7th day and to 0.03 on the 14th day in the intervention group (P<0.001). The severity rates of OM decreased from 1.98 on the 1st day to 1.46 on the 7th day and to 0.62 on the 14th day in the control group (P<0.001). Independent t-test showed no significant difference between the two groups on the 1st day (p = 0.918), while there was a statistically significant difference between the two groups on the 7th (P<0.001) and 14th days (P<0.001, Table 3).
Table 2. Demographic and clinical characteristics of cancer patients undergoing chemotherapy in two groups
| variable | status | Intervention N (%) |
Control N (%) |
p-value |
| Gender | Male | 11(35.5) | 14(45.2) | 0.605 |
| Female | 20(64.5) | 17(54.8) | ||
| Education Level | Illiterate | 8(25.8) | 3(9.7) | 0.644 |
| Elementary | 6(19.4) | 9(29) | ||
| Under high school diploma | 3(9.7) | 10(32.3) | ||
| High school | 10(32.3) | 2(6.5) | ||
| College education | 4(12.9) | 7(22.6) | ||
| Marital Status | Single | 1(3.2) | 1(3.2) | 0.542 |
| Married | 22(71) | 26(83.9) | ||
| Widowed or Divorced | 8(25.8) | 4(12.9) | ||
| Route of administration of chemotherapy agent | Oral | 1(3.2) | 1(3.2) | 0.754 |
| Injection | 30(96.8) | 30(96.8) | ||
| Artificialteeth | Yes | 10(32.2) | 10(32.2) | 0.591 |
| No | 21(67.7) | 21(67.7) | ||
| Age, years( ±SD) | 57.48±14.74 | 58.81±14.13 | 0.720 | |
| Duration of cancer and morbidity (years) | 25.32±20.98 | 18.29±14.59 | 0.131 | |
| Chemotherapy cycles(course) | 2.39±0.66 | 2.32±0.65 | 0.702 | |
Table 3. Comparison of the means of stomatitis severity in the study groups
| Time | Control Mean±Standard Deviation |
Intervention | P-value1 | P-value | ||
| Time2 | time*groups3 | |||||
| 1thday | 1.98±0.51 | 1.97±0.71 | 0.918 | 0.001 |
0.001 |
|
| 7th day | 1.46±1.02 | 0.51±0.57 | 0.001 | |||
| 14th day | 0.62±0.34 | 0.03±0.18 | 0.001 | |||
1. Independent t‑test.2.Effect of time by repeated measure analysis, 3.effect of time and group
Repeated measures ANOVA revealed the effect of time on the severity of OM (P <0.001), indicating a change in its intensity over time in both groups. In addition, the interactive effects of time and group on the severity of OM were statistically significant (P<0.001), indicating a significant difference between the two groups in terms of changes in the severity of OM, where the intervention group experienced a significantly greater reduction in the severity of OM than the control group (Table 3, Figure 2).
Figure 2. Comparison of the means and 95% confidence intervals of severity of stomatitis during the intervention in both groups
Discussion
The present study aimed to evaluate the effect of PSO mouthwash on improvig OM in patients undergoing chemotherapy. The findings showed a greater reduction in the severity of OM in the intervention group using the mixed routine/PSO mouthwash. Reduced severity of OM in the two groups was similar on the first day. However, on the 7th and 14th days, greater reductions were observed in the intervention group compared to the control group.
In line with this finding, Setiadhi et al., (2017) reported that pomegranate seed extract inhibited the growth of recurrent aphthous stomatitis caused by Streptococcus sanguis (25). Khan et al., (2011) also reported that aqueous, ethanol and methanol extracts of pomegranate peel inhibited Staphylococcus aureus, Escherichia coli and Pseudomonas aeruginosa in the disk diffusion test (26). Braga et al., (2005) also confirmed the antibacterial effect of pomegranate extract (27). In other studies, the inhibitory properties of methanol and ethanol extracts of pomegranate peel have been approved on Lactobacillus acidophilus, Streptococcus mutans, Candida albicans (28), Shigella flexneri, Aeromonas hydrophila and oral microorganisms such as Actinomyces viscosus and Lactobacillus fermentum (29).
The current findings can be attributed to tannin, antioxidants and phenolic compounds in different parts of pomegranate. Phenolic compounds present in pomegranate peel and seed exhibit high antibacterial properties (30). Furthermore, different parts of the pomegranate including leaves, bark, seed, root, and juice all contain active ingredients with broad antioxidant activities in addition to antimicrobial properties (27).
Studies have shown that phenolic compounds, especially Punicalagin isolated from pomegranate, have anti-fungal properties against Candida albicans, which causes OM (31). Methanol extract of pomegranate juice and even the gel made from its pericarp (16) has an inhibitory effect against Candida albicans. Therefore, pomegranate extract can be used for the treatment of diseases such as OM, which are caused by various Candida species (32). Consistent with this finding, Vasconcelos et al., (2003) also reported that the use of pomegranate gel turned candida culture of OM negative in people with dentures 48 hours after the completion of the treatment course (33). Other studies also reported the use of pomegranate peel extract in the treatment of Helicobacter (34) and oral pathogens (28).
Pomegranate extract also has antiviral activities against human herpes viruses and human respiratory viruses. Many natural compounds, such as flavonoids, tannins, caffeic acid derivatives, terpenoids, and saponins have antiviral properties against herpes and human respiratory viruses. Antiviral activities of pomegranate extract can be attributed to its high amounts of tannin. It also has antiviral properties against HIV and polioviruses (35).
PSO is rich in phenolic punicalagins, ellagitannins, gallotannin, ellagic acid, gallagic acid, gallic acid and other fatty acids such as anthocyanin, cyanidin, ferulic delphinidin acid and flavonoids, such as flavans, flavanonols, and anthocyanidins (10). These compounds exert a variety of biological activities, such as scavenging free radicals, preventing microbial oxidation and growth, and reducing the risk of cardiac and cerebrovascular diseases and some cancers. Pomegranate seed has antioxidant, anti-tumor, anti-cancer, anti-atherosclerotic and antibacterial properties (16, 30).
It is noteworthy that PSO mouthwash did not cause toxicity, allergic reactions or side effects. In this study, the use of routine mouthwash also reduced the severity of OM. Therefore, a combination of the mouthwash containing PSO, lidocaine, diphenhydramine, Al-mg-s, and nystatin can effectively improve OM.
Conclusion
PSO-containing mouthwash in the intervention group, as compared to the routine mouthwash in the control group, reduced the severity of OM. Given the availability and safety of PSO in comparison with conventional medications, PSO is recommended for the treatment of chemotherapy-induced OM along with existing therapies. Since PSO was used in combination with a routine solution in this study, it is suggested that PSO be used alone in future studies to demonstrate its therapeutic effects. In addition, the effects of aqueous and alcohol extracts of pomegranate seed should be investigated in combination with other mouthwashes in order to achieve a more effective mouthwash.
Among the limitations of this study were small sample size and failure to consider tooth problems (caries, broken teeth, or implants), history of oral diseases, and significant reductions in para-clinical parameters such as white blood cell count and platelet count. It is suggested that further studies check the treatment progress on a daily basis.
Acknowledgements
We sincerely thank the Research Deputy of Kashan University of Medical Sciences and the patients who collaborated in this study (grant NO 9754).
Conflicts of Interest
There are no financial relationships that might lead to a conflict of interest.
Received: 2021/01/10 | Accepted: 2021/10/20 | Published: 2022/08/8
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |







