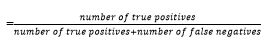BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://journal.zums.ac.ir/article-1-6815-en.html


 , Nasim Namiranian2
, Nasim Namiranian2 

 , Seyyed Kazem Razavi Ratki3
, Seyyed Kazem Razavi Ratki3 

 , Amir Pasha Amlelshahbaz3
, Amir Pasha Amlelshahbaz3 

 , Roghaye Razavi3
, Roghaye Razavi3 

 , Reza Nafisi Moghadam *4
, Reza Nafisi Moghadam *4 


2- Yazd Diabetes Research Center, Shahid Sadoughi University of Medical Sciences, Yazd, Iran
3- Dept. of Radiology, Afshar Hospital, Shahid Sadoughi University of Medical Sciences, Yazd, Iran
4- Dept. of Radiology, Afshar Hospital, Shahid Sadoughi University of Medical Sciences, Yazd, Iran ,
✅ The results of this study show that, owing to the higher sensitivity of chest CT for the diagnosis of COVID-19 pneumonia, performing CT scan for quick diagnosis is recommended for COVID suspected people with negative RT-PCR test results.
Following the outbreak of an unknown type of pneumonia in Wuhan, China, in December 2019, the International Virus Classification Committee identified a new coronavirus from the RNA family of viruses called SARS-CoV-2, which can infect humans (1-3).Then, due to the spread of the disease worldwide, the WHO labeled that pneumonia as a pandemic that could threaten public health and the global economy and as a factor that could destabilize social communities (4-6). Since then, while the symptoms of the disease have been mild to moderate in most patients, some have developed the acute respiratory distress syndrome (ARDS), suffered from multifocal dysfunction, and died (7).
Because the virus can be transmitted rapidly from human to human, it is important to diagnose the disease in the early stages and isolate the infected individuals from the healthy population, thus reducing the transmission of the infection to others in the society and preventing its prevalence (3, 8, 9).To this end, the existence of COVID-19 virus should be checked in patients with common clinical symptoms (e.g.,fever, cough and hypoxemia) (10). The reverse transcription polymerase chain reaction (RT-PCR) is currently the reference standard with very high specificity to identify COVID-19 infection. According to recent reports, the sensitivity of RT-PCR for swab throat samples is limited; it is from 50% to62%, which is too low for screening (11). So, using this method can leave many COVID-19 patients un-identified and, as a result, lead to the rapid transmission of the virus to others (9). Chest CT sensitivity for the diagnosis of COVID-19 has been reported to be high (from 60 to 98%) (12, 13). A previous study showed poor (25%) chest CT specificity for the diagnosis of COVID-19 (9). Overall, studies have shown that, compared to RTPCR, chest CT is of higher sensitivity for detecting COVID-19 (9). As a study found, 3% of the patients had a negative RT-PCR if their chest CT confirmed COVID-19 viral pneumonia (14). As shown in other studies, some patients with positive RT-PCR had normal chest CT. For example, Bernheim et al. reported normal chest CT in 56% of the patients with clinical symptoms (13, 15).In line with these studies, the aim of the present research is to compare the diagnostic values of chest CT and RT-PCR for the patients suspected of COVID-19.
Study design and participants
After the research was approved in the Ethics Committee of Shahid Sadoughi University of Medical Sciences of Yazd (IR.SSU.MEDICINE.REC.1399.131), the purpose of the study and the steps of the work were explained to all the participants, and written informed consent was obtained from them. This cross-sectional study was conducted from March 20, 2020 to June 20, 2020 on all those who had referred to Shahid Sadoughi Hospital in Yazd and were suspected of acute coronavirus 2 respiratory syndrome infection. The clinical symptoms were fever, cough, short breath, body aches and headaches. The patients who did not consent to CT scan after the procedure was explained to them or whose RT-PCR and CT data were not available were excluded from the study.
Chest CT scan protocol and RT-PCR
Over a period of 24 hours or less, 531 patients suspected of COVID-19 underwent chest CT (spiral or HRCT, 1.2 mm incision in inhaling position, TOSHIBA medical systems, Otawara, Japan 16 slices / Alexion) and an RT-PCR test with throat samples. The sensitivity and specificity of the PCR test were calculated in a quadratic table, and the test emerged to be gold standard. The patients' demographic information and the CT scan findings were recorded in the forms designed by the radiologist. The patients were divided into one of the several groups based on the characteristics of their chest CT scan, including the existence of GGO, consolidation, the number of lobes involved, pleural effusion, nodules, lymphadenopathy, and the existence of underlying lung diseases such as emphysema. Then, for the RT-PCR test, the participants were asked to refer to Shahid Sadoughi Medical Genetics Laboratory in Yazd in 24 hours or less. The samples were taken by Dacron swabs and transferred to a respiratory medium for RNA extraction. In the next step, a diagnostic test was performed by the RT-PCR method using a kit (Novel Coronavirus Diagnostic Kit, Sensure Biotech Inc. China). A radiologist who was unaware of the patients' RT-PCR results reviewed the CT scan findings and recorded them in special forms.Then, the RT-PCR data were collected from the electronic medical records based on PCR findings, and the patients were divided into positive and negative PCR groups. The diagnosis of COVID-19 infection was done based on the preliminary chest CT and RT-PCR findings.
Statistical analysis:
The statistical analyze were performed using the SPSS 22 software. The continuous data were displayed as mean ± standard deviation and the stratified variables as number (percentage). Then, a t-test and a chi-square test served to compare the two groups. In this regard, P < 0.05 showed a statistically significant difference.
Demographic characteristics
A total of 531 patients who were suspected of COVID-19 (306 males and 225 females with a mean age of 55.14± 19.7) referred to Shahid Sadoughi Hospital in Yazd, were enrolled in the study according to the inclusion criteria, and underwent chest CT and RT-PCR for 24 hours. Nine patients were excluded from the study because the time interval between their chest CT and RT-PCR test was more than 24 hours, There was not access to the chest CT scans of some patients (N = 6) because they were transferred to other hospitals; Thus, the RT-PCR findings of 522 patients and the chest CT of 506 of them were available for the final analysis. The participants’ demographic characteristics are reported in Table 1.
Table 1. COVID-19 patient’ demographic characteristics as well as initial RT- PCR and Chest CT imaging findings
| Variables | Values | |
| Age, Mean (± SD) | 55.14 (± 19.7) | |
| Sex, N (%) | ||
| Male | 306 (57.6) | |
| Female | 225 (46.4) | |
| Results, N (%) | ||
| Initial RT- PCR assay | + | 291 (55.7) |
| - | 231 (44.3) | |
| Pattern of the initial chest CT imaging, N (%) | ||
| GGO | + | 467 (93.3) |
| - | 39 (7.7) | |
| Patchy consolidation | + | 297 (58.7) |
| - | 209 (41.3) | |
| Strict consolidation | + | 84 (16.6) |
| - | 422 (83.4) | |
| Crazy paving | + | 101 (19.9) |
| - | 405 (80.1) | |
| Reverse hallo | + | 75 (14.8) |
| - | 431 (85.2) | |
| Plural effusion | + | 23 (4) |
| - | 583 (96) | |
| pneumothorax | + | 2 (0.3) |
| - | 504 (99.7) | |
| emphysema | + | 5 (0.9) |
| - | 501 (99.1) | |
| Distribution of the findings in the images, N (%) | ||
| Central | 24 (4.8) | |
| Peripheral | 390 (77.2) | |
| Peripheral/central | 91 (18) | |
- RT-PCR: reverse transcription polymerase chain reaction; GGO: ground glass opacities
CT Findings
As the computed tomography evaluation of the participants showed, the highest values belonged to the GGO and then patchy consolidation groups. These values were obtained through the CT scans of 467(93.3%) and 297(58.7%) patients. In addition, the findings on strict consolidation, crazy paving and reverse hallo were recorded for 84 (16.6%), 101 (19.9%) and 75 (14.8%) patients suspected with COVID- 19, respectively. According to the results, the incidence of complications such as pleural effusion and pneumothorax was very low among the patients. Also, the study of the distribution of the lung lesions on the CT scans of the participants showed that the most involvement was in the peripheral part of the lung (P = 0.002 in 390 of 505 or 77.2% of the cases) (Table 2). Some COVID suspected patients with negative RT-PCR test results had chest CT findings on turbidity [266 of 286], GGO [166 of 286], and patchy consolidation and crazy paving [69 of 286], but the differences were not statistically significant. In terms of negative RT-PCR results and positive reverse hallo findings, however, the participants were statistically different (43 of 286; P = 0.000); Significant differences were also detected in the negative RT-PCR test results and the positive result on strict consolidation (62 of 286; P = 0.001).
Table 2. Comparison of the participants in terms of RT- PCR, chest CT imaging and distribution of lesions
| Parameters |
RT- PCR | P- value |
||||
| - | + | |||||
| Male | Female | Male | Female | |||
| GGO | - | 6 (1.2) | 14 (2.8) | 11 (2.2) | 6 (1.2) | 0.782 |
| + | 111 (22.2) | 155 (31.1) | 90 (18) | 105 (21) | ||
| Patchy consolidation | - | 56 (11.2) | 64 (12.8) | 41 (8.2) | 44 (8.8) | 0.183 |
| + | 61 (12.2) | 105 (21) | 60 (12) | 67 (13.4) | ||
| Reverse hallo | - | 112 (22.4) | 131 (26.3) | 89 (17.8) | 91 (18.2) | 0.000 |
| + | 5 (1) | 38 (7.6) | 12 (2.4) | 20 (4) | ||
| Strict consolidation | - | 102 (20.4) | 122 (24.4) | 93 (18.6) | 97 (19.4) | 0.001 |
| + | 15 (3) | 47 (9.4) | 8 (1.6) | 14 (2.8) | ||
| Crazy paving | - | 91 (18.2) | 126 (25.3) | 93 (18.6) | 91 (18.2) | 0.052 |
| + | 26 (5.2) | 43 (8.6) | 8 (1.6) | 20 (4) | ||
| Central | 10 (2) | 4 (0.8) | 8 (1.6) | 1 (0.2) | 0.002 |
|
| Peripheral | 90 (18.1) | 135 (27.1) | 74 (14.8) | 85 (17.1) | ||
| Peripheral/ Central | 17 (3.4) | 30 (6) | 18 (3.6) | 25 (5) | ||
- RT-PCR: reverse transcription polymerase chain reaction; GGO: ground glass opacities
Comparative diagnostic results
At the beginning of the study, the RT-PCR test results were positive in 291 patients, abnormal CT scan findings were observed in 506 (95.3%) participants, and the chest CT results for COVID-19 were positive in 218 (94.4%) of 231 patients whose RT-PCR result was negative. The diagnostic data obtained from the primary RT-PCR tests and CT scans are reported in Table 3. According to the comparative analysis of the CT scan findings and the initial RT-PCR results, the sensitivity and accuracy of COVID-19 detection with the initial RT-PCR tests was 55.75%, while it was 97.42% with the initial CT scan as for specificity, it was found to be 100% in both initial diagnostic modes. Generally, there was no statistically significant difference between the initial CT detection rate and the initial RT-PCR (P = 0.317).
Sensitivity
Specificity
Table 3. Diagnostic rates of RT-PCR and CT scan
| Diagnostic rate (%) | Sensitivity | Specificity | Accuracy |
| Initial RT- PCR | 55.75 | 100 | 55.75 |
| Initial CT imaging | 97.42 | 100 | 97.42 |
| P- value | 0.0001 |
1.000 | 0.0001 |
Discussion
According to the literature on COVID-19, the most important measure to control and prevent the spread of the disease is the rapid diagnosis of the infected individuals, their rapid isolation and quarantine, and the identification and isolation of all those in close contact with them from the healthy population (16). In this regard, RT-PCR is of relatively low diagnostic sensitivity, has limited diagnostic kits, and is practiced with insufficient skills in many countries. Indeed, it fails to identify many people infected with COVID-19, which leads to further transmission of the virus (11). Therefore, this method can-not be the only reliable tool for COVID-19 screening (11).
In this study, the PCR test was positive for 291 patients infected with COVID-19, but the chest CT of many participants showed a variety of findings on variables such as GGO, patchy consolidation, and peripheral distribution of lesions. As expected, the most common of these findings was GGO, and the rarest were pleural effusion and pneumothorax. According to the statistical data, the CT scans of the positive and negative PCR groups were significantly different only in terms of reverse hallo data and lesion distribution location. However, GGO lesions and patchy consolidation were not significantly different in the two groups.
The diagnostic sensitivity of the RT-PCR test in this study was 55.75%; the results here are in line with the findings of some other studies in this field, such as Ai et al. (9). In a study of 82 hospitalized patients conducted by He et al., the sensitivity and specificity of RT-PCR was found to be 79% and 100%, respectively (11). This difference can be due to some factors such as the differences in the distribution of the kits used, the method and quality of testing, and the viral load on the respiratory tract (17).
In the present study, the diagnostic sensitivity of the chest CT test conducted on COVID-19 patients was 97.42%. In a similar study on 36 COVID- 19 pneumonia- suspected patients conducted by Long et al. the CT sensitivity of the chest emerged to be 97.2%, which confirms the CT scan findings obtained in this study. In terms of the PCR sensitivity, however, Long et al. (ibid) obtained a value of 83.3%, which is higher than that in the present study (3). The sensitivity of imaging calculated by Hao et al. is consistent with the results of our investigation (18). Also, Dangis et al. evaluated the diagnostic sensitivity, specifity and accuracy of the CT scans of 192 COVID- 19 patients. Those factors were 86.7%, 93.6% and 91.1% respectively. In another study on symptomatic patients for 48 hours, the sensitivity, specificity and accuracy of the CT scans were 95.6%, 93.2% and 91.5%, respectively (19). Moreover, in a study in 2020, the sensitivity and specificity of chest CT were reported to be 77% and 96%, respectively (11). In a large study on 919 patients in Wuhan, the chest CT sensitivity was 97% (9). Generally, the chest CT sensitivity in studies for the diagnosis of COVID-19 has been up to 98% (20). This suggests that chest CT imaging, due to its high sensitivity, easy access and cost-effectiveness, can serve as an effective diagnostic method to separate COVID-19 suspected people from others (11).
The findings of this study point to a statistically significant difference between chest CT and RT-PCR in terms of sensitivity. Fang et al. demonstrated that chest CT was significantly more sensitive than RT-PCR (98% and 71%, respectively) (20), which is in line with the findings of our study. In the study conducted by He et al., the difference of the two techniques was not significant (11). In the same vein of research, Bai et al. found no significant relationship between rt-PCR and chest in terms of sensitivity and specificity (21).
In a study in Tabriz, Portahmasebi et al. concluded that CT scan is suitable for assessing the disease and its severity, but it is not recommended for screening COVID- suspected ones because of the harmful ionizing radiation used in it (8). In this respect, some studies have shown that at limited dose of radiation in chest CT does not cause cancer (22); Anyway, it is suggested that this method be used with caution for certain age groups and vulnerable people.
In an extensive study by Yang et al., several pieces of research with various statistical populations were reviewed and analyzed for their findings, which is worth considering. It was found that, in many cases, normal CT scans had been done on the early days of the disease despite positive PCR test results. This may be due to the fact that COVID- infected people in areas outside the epidemic have only the initial and mild symptoms of the disease. As a result, there is no pneumonia or lung involvement in these cases, and their chest CT scan is normal (11, 14). Herein, it is suggested that further studies be designed with larger size samples, over longer periods of time, in several medical centers and by using gold standard diagnostic methods to yield more reliable and helpful results.
Conclusion
As shown by many studies in the short time since the onset of the COVID- 19 pandemic, the early diagnosis of the disease and, consequently, the disruption of its transmission chain are among the major concerns of all medical systems in the world although there are many controversies over procedures and findings. Overall, the results of this study suggest that RT-PCR may be negative in many COVID-19-infected individuals, while chest CT scans are more sensitive for the detection of the virus as well as faster and more accurate for the evaluation of those patients. Hence, due to the higher sensitivity of chest CT for the diagnosis of COVID-19 pneumonia, it is recommended that people who are clinically suspected of COVID-19 but have a negative RT-PCR test be evaluated for a quick diagnosis of infection using a chest CT scan. If a person proves to be suspected of COVID-19 pneumonia on a chest CT scan, it is necessary to isolated him or her from other people in the society and to start appropriate treatment to prevent further infection or outbreak of the disease.
Acknowledgements
The authors thank all the study participants and the hospital staff for their support and cooperation in this study.
Funding
This research was financially supported by the Vice Chancellor of Research at Shahid Sadoughi University of Medical Sciences and Health Services in Yazd.
Conflicts of Interest
The authors declare no conflict of interests regarding this study.
Received: 2022/08/8 | Accepted: 2023/01/20 | Published: 2023/06/26
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |