BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://journal.zums.ac.ir/article-1-6931-en.html


 , Afshin Sarraf1
, Afshin Sarraf1 

 , Mandana Khodashahi1
, Mandana Khodashahi1 

 , Maryam Sahebari1
, Maryam Sahebari1 

 , Lida Jarahi2
, Lida Jarahi2 

 , Hamid Reza Rahimi3
, Hamid Reza Rahimi3 

 , Shima Nabavi *4
, Shima Nabavi *4 


2- Dept. of Community Medicine, Faculty of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran
3- Dept. of Modern Medical Sciences and Technology, Faculty of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran
4- Dept. of Internal Medicine, Faculty of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran ,
✅ The present study revealed that curcumin had no significant therapeutic effects on reducing the activity of RA; however, no significant side effects were observed on the patients, and it also showed its analgesic effect well.
Rheumatoid Arthritis (RA) is a chronic symmetric inflammatory disease with unknown etiology. It is considered the most common form of chronic arthritic inflammation associated with articular and extra-articular manifestations (1, 2). Generally, RA is observed in about 0.5%-1% of the world’s adult population. Furthermore, this disease commonly occurs in the fourth to fifth decades of life, and in 80% of the cases, at the ages of 35 to 50 years (3, 4).
The control over the joint symptoms, prevention of cartilage destruction, and maintenance of the joint movements in patients with RA require continuous treatment. Accordingly, treatment cessation often leads to the relapse of the disease symptoms (5). The treatment options for RA include non-steroid anti-inflammatory medications, glucocorticoids, disease-modifying anti-rheumatic drugs (DMARDs), and biological medicines (6).
DMARDs slow the progression of joint damage and reduce the immune responses in RA patients. The use of the treatment could improve pain and inflammation in these patients. Synthetic DMARDs is differentiated into conventional and targeted therapies. According to Smolen et al., conventional synthetic DMARDs were not developed based on a specific mechanism of action. Synthetic DMARDs have been divided into two categories of targeted synthetic DMARDs and conventional synthetic DMARDs in the new terminology (7).
In RA, the roles of small-molecule mediators of inflammation (e.g., arachidonic acid metabolites), cytokines, tumor necrosis factor α (TNF-α), chemokines, adhesion molecules, and matrix metalloproteinases have been carefully demonstrated (8-10). The TNF-α is a key mediator of inflammation in RA, and this influence is controlled by the activation of nuclear factor (NF)-κB. Thus, agents that downregulate NF-κB and NF-κB-regulated gene products can function against RA potentially. A recently developed anti-inflammatory medication for suppressing synovial and systemic inflammation in RA is curcumin, whose high efficacy has been shown in various studies (11, 12). Curcumin as an anti-inflammatory medicine has been shown to block NF-κB activation by various inflammatory stimuli (13-15). Further, it is safe and non-toxic and shows its anti-inflammatory effects by regulating the inflammatory factors of cytokine transcription, as well as the redox status of protein kinase plus enzymes that all increase inflammation. Curcumin also causes apoptosis through the mitochondrial and receptor-mediated pathways, as well as the activation of caspase (16). Regarding the low toxicity of curcumin and the excellent tolerance of the body against it, the use of this substance is increasing in the treatment of various types of diseases (17).
The anti-inflammatory effects of curcumin on the inactivation of free radicals and its anticancer effects have been proven so far, mainly due to its effect on cell enzymes, cytokine transcription, protein kinase, inhibition of signaling pathways at different levels, angiogenesis, and cell adhesion. Given the effects of curcumin on gene transcription and induction of apoptosis, it seems that this medication can be used for the treatment of various diseases. However, it should be noted that these effects are often dose-dependent and contingent upon the environmental conditions (11).
The use of curcumin as a safe and non-toxic pharmaceutical agent is promising in the treatment and prevention of cancers as well as autoimmune-inflammatory diseases (18). However, there have been incomplete studies evaluating the therapeutic and side effects of curcumin on the treatment of debilitating diseases, such as RA. Accordingly, there is a need for more precise experiments to understand the impact of mechanisms and estimate the effective doses. With this background in mind, this study aimed to investigate the anti-inflammatory effects of curcumin on RA.
This double-blind randomized clinical trial included 64 patients with RA, who were referred to the Rheumatology Research Center of Ghaem Hospital affiliated to Mashhad University of Medical Sciences, Mashhad, Iran, from 2017 to 2018.
Inclusion and Exclusion Criteria
The inclusion criteria were: 1) RA diagnosis by a rheumatologist according to the European League Against Rheumatism/American College of Rheumatology (ACR) criteria, 2) age range from 18 to 75 years, and 3) RA with Disease Activity Score (DAS)-28 erythrocyte sedimentation rate (ESR)>2.6. Meanwhile, the exclusion criteria included: 1) the use of biologic medications, such as Etanercept (receptor of TNF-α solution), infliximab, anti-TNF-α antibody, and Anakinra (antagonist protein, a receptor of interleukin-1), 2) the use of medications that inhibit the immune system (Azathioprine, Cellcept, Tacrolimus, Leflunomide, Endoxan, Sulfasalazine, and Hydroxychloroquine), 3) the use of Pentoxifylline, Cilostazol, and supplements affecting RA (extract of Berberis Vulgaris [berberine]), glucosamine, and Piascledine, and 4) the concomitant use of prednisolone more than 10 mg per day, the use of NSAID, and treatment with intramuscular and intra-articular corticosteroids (more than 7.5 mg per day) during the previous two weeks to day zero. Moreover, the patients treated with intravenous, intramuscular, and inedible corticosteroids within recent two weeks or intra-articular corticosteroids within recent four weeks were excluded from this study. Furthermore, a history of sensitivity to curcumin (turmeric), renal failure (through checking GFR <60), and hepatic failure (through checking ALT and AST liver enzymes higher than three times the normal value and direct bilirubin greater than 0.2 and total higher than 2), significant extra-articular involvement (Felty's syndrome and vasculitis), history of diseases causing defective intestinal absorption syndromes (according to patient’s history), breastfeeding and pregnancy, active peptic ulcers, other autoimmune diseases (overlapping with other collagenous diseases), newly-diagnosed patient with RA, and a high degree of disease activity (DAS28-ESR> 5.1) were the other exclusion criteria.
Study Design
The sample size was determined 29 cases in each group based on previous studies using a power test of 90% and a two-sided significance level of 5%. However, considering the sample attrition, 32 cases were assigned into each group. The patients were randomly assigned into two groups of intervention (group A) and placebo (group B) using the sequence numbers.
A researcher-made questionnaire was used to collect the patients' information (n=64). Note that the participants, researchers, and the assessor were unaware of the assigned intervention. Following that, an independent researcher made random allocation cards using computer-generated random numbers and offered random sequences to the main researchers. Furthermore, another independent researcher prepared the medications for “group A” (curcumin nano-micelle with a brand name of Sina Curcumin) and “group B” (placebo in the form of nano-micelles with a brand name of Sina Company) and put them into envelopes according to the allocation orders. After the end of the analysis, groups A and B were informed about the therapeutic procedure by the independent researcher.
The patients in the intervention group consumed 80 mg curcumin nano-micelle after lunch daily along with their standard treatment of RA. On the other hand, the controls consumed placebo after lunch daily along with the routine treatment. During the three months of the study, there was no change in the routine and standard drug regimen of the patients in the intervention and control groups. The regimen included daily prednisolone and weekly methotrexate (according to previous administration), 1 mg of folic acid daily, 500 mg of calcium-D daily, and 70 mg of alendronate weekly. During the study, the number of medications used was not changed as far as possible, and the need for NSAIDs was recorded at examination intervals. Note that the patients were supposed to have no changes in the dose of methotrexate and prednisolone during three weeks. The treatment duration was 2-39 months for all patients. The use of NSAID was forbidden to use, except for during severe pain. The exact duration of receiving methotrexate and corticosteroids in the recent months has not been determined. However, the patients' situation has been stable over the last two or three months and the dosage of methotrexate and corticosteroids had not increased or decreased, recently.
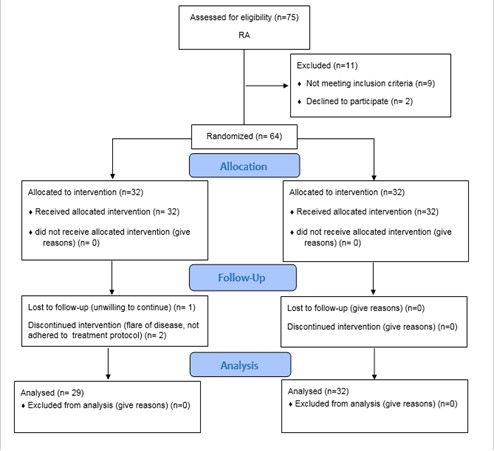
In case of using analgesics, it was recorded 48 h before the examination. During the study, there were three dropouts due to disease flareup (n=1), unwillingness to continue the study (n=1), and failure to follow the defined treatment protocol (n=1) (Figure 1).
Regarding the lipophilic nature of curcumin, the absorption of capsules, powder, and pills is very low. SinaCurcumin® oral soft gelatin capsules (Exir Nano Sina Company, Tehran, Iran) were used in this study to improve the oral bioavailability of curcumin. SinaCurcumin® is a registered product from curcuminoids in Iran (IRC: 1228225765) (19). Curcuminoids are dietary polyphenols extracted from the dried rhizomes of Curcuma Longa (Turmeric) and are composed of curcumin (79.4%), desmethoxycurcumin (17.6%), and bisdemethoxycurcumin (3%), which altogether are known as the C3 complex. Each soft gelatin capsule of SinaCurcumin® contains 80 mg curcuminoids as nano-micelles. The encapsulation efficiency of curcuminoids in nano-micelles is near 100%, with the mean particle diameter obtained at around 10 nm (20).
Efficacy and safety assessment
The patients in both intervention and control groups were assessed for three months at one-month intervals. Furthermore, the scores obtained from the disability index of the health assessment questionnaire, clinical laboratory examination results according to DAS-28, assessment results of the incidence of adverse effects, and the amount of required NSAID in the previous month were recorded in the forms. Note that a rheumatologist performed all of the aforementioned assessments.
Statistical analysis
The data obtained from the demographic characteristics and clinical observations of the patients in both groups were analyzed by SPSS software (version 22). Furthermore, descriptive statistical methods, including central tendency, dispersion, and frequency distribution were used to describe the data. The Mann-Whitney U test was also employed to compare the effects of the intervention on the treatment procedure in both groups during the study. The trend of changes in the indicators during the study intervals in the intervention and control groups was evaluated using the Friedman nonparametric test. A p-value less than 0.05 was considered statistically significant.
Regarding the ethical consideration, the participants were assured of the confidentiality of their information during and after the study. Further, informed consent was obtained from all patients. They were also assured that they could leave the study at any time if they were unwilling to continue their participation in the study or follow-ups. The study protocol was approved by the Organizational Ethics Committee of Mashhad University of Medical Sciences (IR.MUMS.REC.1394.303) (No. 5621537). It was also registered in the Iranian Clinical Trial Registry system (IRCT20170812035630N1).
A total of 64 patients participated in this study and were divided into two groups of intervention and control. In total, three cases from the control group were excluded due to disease flare, lack of willingness to continue the study and intervention, and lack of suitable drug compliance (Figure 1).
Based on the obtained results, the mean age of each group was obtained at 55 years (age range: 22-71 years) (P=0.78), and the majority of the patients in the intervention (90.3%) and control groups (90.9%) were female (P=0.9). Further, the cyclic citrullinated peptide (CCP) antibodies were reported in 59.2% and 40.7% of the cases in the intervention and control groups, respectively (P=0.21). There was no significant difference between the intervention and control groups in terms of the demographic characteristics, such as age, gender, and positive anti-CCP before the onset of the study.
The evaluation of laboratory parameters before the onset of the study demonstrated no significant difference between the two groups in this regard, and they were comparable in terms of undergoing the intervention (Table 1). In addition, no difference was observed between the two groups in terms of anti-CCP, ESR, DAS-28, patient pain score, global health assessment, and physician assessment (Visual Analog Scale [VAS]) variables before the intervention. It was well shown that the random allocation of the subjects in the two groups had been performed accurately. The results of evaluating the treatment outcomes at the end of the study in both intervention and control groups are presented in Table 2.
As can be observed in Table 2, there is no significant difference between the two groups in terms of some evaluation indicators of disease activity (e.g., number of painful joints, physician assessment (VAS), disability index, ESR, and global health assessment). However, a significant difference was observed between the intervention and control groups regarding the pain scores of the subjects (P<0.05). Indeed, the intervention group obtained a lower mean pain score, compared to the control group at the end of the treatment. The number of swollen joints in the intervention group at the beginning and end of the treatment as well as intervention with curcumin also showed a significant difference in this regard. Accordingly, the mean numbers of swollen joints were 2 and 5 in the intervention and control groups at the end of the treatment, respectively (P<0.05).
Additionally, a significant difference was found between the groups regarding the changing trends of ESR, disability index, patient pain assessment, physician assessment (VAS), and the number of tender and swollen joints (P<0.05) which could be due to the therapeutic outcomes of using the standard medications in the current state, which led to recovery and remission. In general, 46.67% (7 cases) and 53.33% (8 cases) of patients in the intervention and control groups received analgesics, respectively. A high level of DAS28 was observed in patients who took analgesics, while a low level of DAS28 was reported in those who did not receive analgesics. There was no significant difference between the two groups in terms of the consumption of analgesics (P=0.71). The comparison of DAS-28 score (P=0.11), pain score (P=0.04), and swollen joints (P=0.02) between the two groups at different time points is shown in Figures 2 and 3. No significant side effects (e.g., nausea, vomiting, diarrhea, or other complications) were noted in any of the patients in the control and intervention groups.
Table 1. Baseline characteristics of the subjects in the control and intervention groups at the beginning of the study and before the intervention
| Laboratory findings | Control group (n=29) | Intervention group (n=32) | P-value | ||
| Mean | SD | Mean | SD | ||
| White blood cell (×109 cells/L) | 7.6 | 2.2 | 7.08 | 1.7 | 0.53 |
| Red blood cells (×109 cells/L) | 4.6 | 0.3 | 4.4 | 0.3 | 0.1 |
| Aspartate aminotransferase (U/L) | 18.8 | 5.1 | 18.2 | 5.5 | 0.59 |
| Alanine aminotransferase (U/L) | 19.3 | 9.5 | 17.9 | 7.1 | 0.78 |
| Alkaline phosphatase (U/L) | 173.3 | 43.3 | 172 | 51.7 | 0.99 |
| Bill total (mg/dL) | 0.75 | 0.22 | 0.64 | 0.21 | 0.06 |
| Bill direct (mg/dL) | 0.2 | 0.07 | 0.2 | 0.06 | 0.54 |
| Hemoglobin (g/dl) | 13.1 | 1.4 | 12.9 | 1.2 | 0.36 |
| Hematocrit (L/L) | 40.1 | 3.8 | 39.4 | 3.2 | 0.36 |
| Polymorphonuclear neutrophils | 61.1 | 7.8 | 60.8 | 11.1 | 0.702 |
| Lymphocyte count | 31.2 | 7.5 | 32.4 | 9.3 | 0.46 |
| Platelet count | 285.3 | 57.9 | 273.9 | 75.3 | 0.27 |
| Blood urea nitrogen (per liter) | 14.9 | 3.7 | 5.1 | 2.9 | 0.507 |
| Creatinine (mg/dL) | 0.91 | 0.16 | 0.89 | 0.19 | 0.41 |
| Triglycerides (mg/dL) | 129.1 | 46.5 | 114.8 | 48.3 | 0.16 |
| Cholesterol (mg/dL) | 114.9 | 31.2 | 112.3 | 30.5 | 0.61 |
| Fasting blood sugar (mg/dL) | 94.03 | 18.1 | 92.9 | 12.8 | 0.47 |
| MTX (mg)/week | 13.3 | 3.6 | 11.8 | 3.7 | 0.108 |
| Pred (mg)/day | 5.9 | 2.2 | 5.1 | 1.2 | 0.14 |
Table 2. Comparison of intervention and control groups at follow-up regarding the evaluation of disease activity indicators
*Significance level less than 0.05
| Indicators | Before intervention | P-value | 1 month after intervention | P-value | ||
| Intervention | Control | Intervention | Control | |||
| Tender | 3 (1-6) | 3 (1-6) | 0.79 | 1 (0-3) | 2 (1-5) | 0.17 |
| Swollen | 4 (1-6) | 4 (1-6) | 0.11 | 2 (2-4) | 4 (2-6) | 0.02* |
| Physician assessment | 3 (2-4) | 4 (2-5) | 0.36 | 2 (2-3) | 3 (2-4) | 0.27 |
| Global health assessment | 38.06±18.6 | 40.9±20.05 | 0.46 | 32.9±18.6 | 33.9±23.9 | 0.77 |
| DAS-28 | 4.2±0.78 | 4.25±0.8 | 0.75 | 3.8±1.1 | 3.9±1.05 | 0.7 |
| Disability index | 0.83±0.56 | 0.79±0.61 | 0.36 | 0.78±0.49 | 0.74±0.59 | 0.48 |
| Pain score | 38.3±23.8 | 43.3±22.3 | 0.406 | 35±22.4 | 35.9±22.7 | 0.86 |
| ESR | 29.22±18.8 | 25.3±15.4 | 0.36 | 27.7±16.5 | 24.7±19.02 | 0.23 |
| Indicators | 2 months after intervention | P-value | 3 months after intervention | P-value | ||
| Tender | 1 (0-3) | 2 (1-4.5) | 0.38 | 1 (0-3) | 1 (0-3) | 0.58 |
| Swollen | 3 (2-4) | 3 (2-5.5) | 0.44 | 2 (1-3) | 3 (2-4) | 0.02* |
| Physician assessment (VAS) | 2 (1-3) | 2.5 (1-3) | 0.28 | 2 (1-2) | 1 (1-3) | 0.93 |
| Global health assessment | 27.09±18.8 | 37.8±30.9 | 0.28 | 22.5±20.6 | 24±19.9 | 0.65 |
| DAS-28 | 3.7±0.8 | 3.7±1.2 | 0.62 | 3.6±1.04 | 3.2±1.07 | 0.11 |
| Disability index | 0.64±0.46 | 0.84±0.8 | 0.82 | 0.55±0.38 | 0.72±0.61 | 0.406 |
| Pain score | 28.8±23.01 | 34.6±23.1 | 0.2 | 20±22.2 | 27.5±20.3 | 0.045* |
| ESR | 25.9±12.4 | 19.3±15.1 | 0.056 | 26.4±12.5 | 18.7±14.2 | 0.12 |
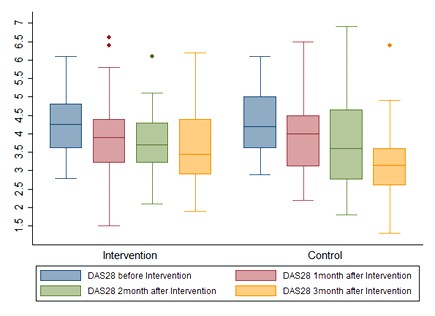
Figure 2. DAS-28 score of the subjects by the group at time points of the study
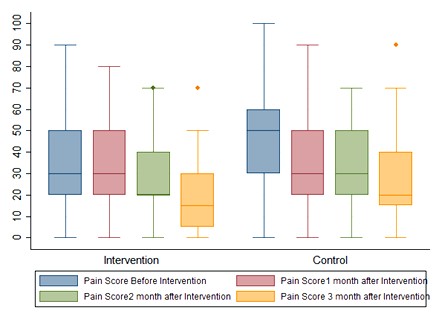
Figure 3. Pain score of the subjects under study in the intervention and control groups at time points of the study
Discussion
The intervention group obtained a lower mean pain score at the end of the treatment, compared to the control group. Further, there was a significant difference between the two groups in terms of the changing trends of ESR, disability index, patient pain assessment, physician assessment (VAS), and the number of tender and swollen joints. However, no difference was noted between the two groups regarding some evaluation indicators of disease activity and the number of painful joints.
Curcumin has a therapeutic potential for various chronic diseases, in which inflammation plays an important role. According to epidemiological studies, the prevalence of chronic diseases is lower in countries where people consume curcumin (17, 21). Evidence from various studies indicates the role of curcumin in inhibiting the proliferation, invasion, and angiogenesis of inflammatory cells, which show their effects through molecular mechanisms (16, 22, 23). Since curcumin inhibits several pro-inflammatory pathways and is also cost-effective, it is necessary to further investigate this substance for the prevention and treatment of various chronic diseases.
The results of this double-blind randomized clinical trial revealed that the administration of curcumin along with prednisolone and methotrexate was safe in patients with mild to moderate RA and had no side effects. Additionally, it showed no significant effect on the ESR-DAS-28 index. However, the patients' assessment of their pain and the number of swollen joints in the intervention group showed a significant decrease, compared to the control group.
Other ACR components (i.e., number of painful joints, physician assessment [VAS], ESR, and disability index) showed no significant difference in the two groups. Nonetheless, in a randomized three-arm trial conducted by Chandran (5) on patients with RA, curcumin alone or in combination with diclofenac sodium improved DAS-28 and ACR indicators in patients with mild or moderate RA, compared to the group treated with diclofenac alone. Further, all ACR components, including the number of swollen and painful joints, physician assessment (VAS), patient disability index, and inflammatory index (C-reactive protein [CRP] in that study) diminished significantly. This observed discrepancy between our results and those in the aforementioned study could be due to the discontinuation of methotrexate as DMARD in our study in both the intervention and control groups; however, Chandran used NSAID for the control group. Regarding the pain reduction reported by the patients and the number of swollen joints at the end of the study, our results were consistent with the findings of the study performed by Chandran.
Several studies have investigated the effect of curcumin on the reduction of chronic pains (24-27). Furthermore, curcumin was effective in reducing arthritis pains associated with osteoarthritis (3), which was in line with the results of our study in terms of pain relief. In a study carried out by Cheol Park, it was found that curcumin stimulated apoptosis in synovial fibroblasts of RA patients, and therefore, had anti-proliferative effects. Curcumin simultaneously lowered the release of prostaglandins as well as inflammatory mediators plus pain, while reducing the incidence of COX2 (28). It seems that the reduction in the number of swollen joints in the intervention group is consistent with the results of the present study.
Although numerous studies have indicated the anti-oxidant, anti-inflammatory, and anti-cancer effects of curcumin (21), it seems that its low absorption and low bioavailability have limited its effect on human models. In a study performed by Douglass (29), the bioavailability of six different curcumin formulations was compared and a significant difference was found in this regard. In the same vein, the results of a study conducted by Amalraj (30) on patients with active RA who had stopped taking their routine rheumatoid drugs within four weeks revealed that the administration of curcumin in the three arms over three months with doses of 250 and 500 twice a day had a significant effect on reducing clinical symptoms, ACR response, DAS-28, physician assessment (VAS), ESR, and CRP, compared to the placebo group. Further, no toxic side effect was observed at those dosages. The observed difference in the results of this study seems to be related to the different bioavailability of the curcumin combination used, as well as the discontinuation of DMARD and corticosteroid in that study. Although the number of subjects in this study was sufficient for a statistical conclusion, further studies are recommended to be conducted with a larger sample size and investigate different curcumin formulas in terms of bioavailability.
This study was the first attempt assessing the effects of oral curcumin on RA patients in Iranian population. The oral use of curcumin to the treatment of anti-inflammatory problems in RA patients was the other innovation of the study, which can be a way to conduct more studies in this field.
Advantages and Limitations
This double-blind randomized clinical trial aimed to investigate the effect of curcumin in combination with other standard RA medications on disease activity and quality of life in patients with RA. The homogenous sample of patients and prospective nature of the study are the main strengths of our study. However, the limitations include the small sample size and the lack of curcumin assessment in different dosages. Further studies are suggested to be conducted with larger samples to validate our results.
Conclusion
The present study indicated that curcumin had no significant therapeutic effects on decreasing the activity of RA. However, no significant side effects were observed on the patients, and its analgesic effect was also confirmed in this study.
Authors' Contributions:
This work was carried out in collaboration among all authors. ZRY designed the study, LJ performed the statistical analysis, SN, MS wrote the protocol and managed the literature searches MKH wrote the first draft of the manuscript. AS and HR managed the analyses of the study. All authors read and approved the final manuscript.
Acknowledgements
The authors of this article gratefully acknowledge the vice-president for research in Mashhad University of Medical Sciences and the Rheumatology Research Center of Ghaem Hospital as well as all the patients for their sincere cooperation. Dr. Jafari is also greatly appreciated for his valuable guidance and drug preparation for this study.
Funding
None.
Conflicts of Interest
The authors declare that there is no conflict of interest.
Received: 2022/08/18 | Accepted: 2022/12/26 | Published: 2023/10/29
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |