BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://journal.zums.ac.ir/article-1-7484-en.html


 , Angela Hamidia2
, Angela Hamidia2 

 , Azadeh Ramezani3
, Azadeh Ramezani3 

 , Saeed Abrtan3
, Saeed Abrtan3 

 , Hoda Shirafkan4
, Hoda Shirafkan4 

 , Hirbod Hadizadeh5
, Hirbod Hadizadeh5 

 , Negin Tavakoli5
, Negin Tavakoli5 

 , Romina Hamzehpour *6
, Romina Hamzehpour *6 


2- Department of Psychiatry, Social Determinants of Health Research Center, Babol University of Medical Sciences, Babol, Iran
3- Department of Psychiatry, School of Medicine, Babol University of Medical Sciences, Babol, Iran
4- Social Determinants of Health Research Center, Health Research Institute, Babol University of Medical sciences, Babol, Iran
5- Student Research Committee, Babol University of Medical Sciences, Babol, Iran
6- Department of Psychiatry, Social Determinants of Health Research Center, Babol University of Medical Sciences, Babol, Iran ,
Heart failure (HF) is a complex and multifactorial clinical syndrome caused by structural or functional disorders of the heart that impair its ability to fill with or eject blood (1). This results in various clinical symptoms, including shortness of breath, fatigue, rales, and edema (2). The severity of HF is classified into four stages according to the New York Heart Association (NYHA) classification system (3), with this condition affecting approximately 6-10% of individuals over the age of 65 (4).
In recent years, depression has gained significant attention in HF research, prompting increased vigilance among healthcare providers to identify and screen for depression in this population (5). A meta-analysis reported that the prevalence of depression in HF patients ranges from 11% to 45% (6). In a pooled analysis of 36 studies, 21.5% of HF patients exhibited clinically significant depression, with one-third of these patients showing elevated scores on the Hamilton Depression Rating Scale (HDRS) (7). Depression in HF patients has been identified as a key risk factor for increased morbidity and mortality, affecting self-care abilities and overall quality of life (8). In addition to psychological factors, particularly depression, heart transplantation outcomes are also markedly impacted by depression, with poorer outcomes linked to depression, as well as reduced self-care abilities (9).
The prevalence of depression and anxiety in HF patients is associated with several adverse outcomes, including poor treatment adherence, reduced functional capacity, increased hospitalizations, and higher mortality rates (9). Despite the high burden of these conditions, depression and anxiety often go undiagnosed and undertreated in HF patients (10). Furthermore, meta-analysis findings suggest that the prevalence of depression in older adults is similar across different countries. However, the availability of mental health resources, especially in low- and middle-income countries, remains limited, exacerbating the treatment gap (11). A study involving 396 HF patients found that 21% were clinically depressed, with 60% receiving antidepressants (12). Depression is a significant independent predictor of all-cause mortality in HF patients, and it increases the risk of hospitalization and readmission. Notably, depression can double or triple the one-year mortality risk in patients with advanced HF (NYHA Class III and IV) (8, 13).
Agomelatine is a novel antidepressant that acts as an agonist at melatonin type 1a and 1b receptors and an antagonist at the 5-hydroxytryptamine 2C (5-HT2C) serotonergic receptors (14). It is known for its antidepressant effects, sleep-promoting properties, and ability to regulate circadian rhythms (15). These characteristics make Agomelatine a promising option for treating depression in HF patients, mainly due to its potential to address polypharmacy concerns commonly faced by this population (16). Unlike traditional antidepressants such as Sertraline, Agomelatine may offer distinct advantages in managing both depression and associated sleep disturbances in HF patients (6, 17, 18).
While Agomelatine has shown promise in the treatment of depression in other populations, there is limited research on its effectiveness in HF patients. This study aims to compare the effects of Agomelatine with Sertraline, a widely used antidepressant, in treating depression in HF patients. Additionally, the study will examine the side effects of both medications, providing valuable insight into their comparative safety profiles in this patient group. This study fills a critical research gap and guides treatment decisions for this vulnerable population in light of the high prevalence of depression in HF patients, particularly among the elderly.
2.1 Study Population
A comprehensive medical history was obtained for all patients, covering symptoms, personal and family history, and a thorough clinical examination. Additionally, patient medical records were reviewed to ensure eligibility. Structured questionnaires were used in data collection, which consisted of two sections:
- Personal and Demographic Information: Questions capturing individual and demographic details of the patients.
- Depression Assessment: Questions derived from the Beck Depression Inventory-II (BDI-II) and the Hamilton Depression Rating Scale (HAM-D).
- Sertraline Group: Treatment began with 25 mg daily (half a tablet, manufactured by Sobhan Pharmaceuticals). Gradually, the dose was increased to 200 mg based on the patient's response and tolerability (20).
- Agomelatine Group: Treatment started with 25 mg daily (one tablet, manufactured by Takajeh Pharmaceuticals). Based on clinical response and side effects, the dose was titrated to a maximum of 50 mg (21).
2.2 Sample Size Calculation
The sample size was calculated using G*Power software for a mixed ANOVA repeated-measure within-between interaction. Based on similar studies, an effect size of 0.25 was chosen. With a significance level (α) of 0.05, a power of 80%, and four measurement time points, the minimum sample size required was 24 participants per group. The study included 58 patients in order to account for an estimated 20% dropout rate.
2.3 Study Design
This randomized, double-blind clinical trial assigned participants to two groups in a 1:1 ratio: one receiving Agomelatine and the other Sertraline. The study complied with the CONSORT guidelines.
2.4 Inclusion and Exclusion Criteria
2.4.1 Inclusion Criteria
- Age ≥18 years.
- Diagnosed with heart failure.
- Symptoms of depression, as indicated by a BDI-II score >14 and a HAM-D score >7.
- Cognitive impairment or mental retardation.
- History of substance abuse or bipolar disorder.
- Kidney or liver failure.
- Any uncontrolled medical condition.
- Assessment Tools
It includes 21 items that assess depression severity on a Likert scale of four points. Based on scores ranging from 0 to 63, the categories are as follows:
-
- 0–13: Minimal depression.
- 14–19: Mild depression.
- 20–28: Moderate depression.
- 29–63: Severe depression.
Clinicians administered a 17-item tool to assess depression symptoms. Score categories are:
-
- <7: No or minimal depression.
- 7–17: Mild depression.
- 18–24: Moderate depression.
- ≥25: Severe depression.
The patients were divided into two treatment groups:
Agomelatine Group: Patients were prescribed 25 mg daily, and the dose was titrated up to 50 mg depending on their clinical response and side effects.
Sertraline Group: Patients began with half a 25 mg tablet daily, with the dose gradually increased to a maximum of 200 mg based on treatment response and tolerability.
Randomization was performed using block randomization with a 1:1 allocation ratio. Quadruple blocks (e.g., AABB, ABAB) were used to ensure balanced group assignment. A statistical advisor not involved in data collection generated the randomization sequence using Random Allocation Software (RAS) version 9.4. Sealed envelopes containing group assignments were sequentially opened after enrolling every four patients.
To ensure a double-blind design:
- The researcher administering the questionnaires was blinded to treatment assignments.
- The psychiatrist prescribing medications was blinded to questionnaire results.
- Both medications were identically packaged to prevent recognition of treatment groups by patients or study staff.
2.7.1 Primary Outcome
Depression scores were assessed using the BDI-II and HAM-D at baseline, and at weeks 4, 8, and 12.
2.7.2 Secondary Outcome
Side effects were monitored using a structured checklist, developed by the researcher and based on patient reports during follow-up visits.
-
- Statistical Analysis
Comparative Analysis:
-
- Independent t-tests were used to compare continuous variables between groups.
- Chi-square or Fisher's exact tests were used for categorical variables.
Longitudinal Analysis: Mixed ANOVA was conducted to evaluate changes in depression scores over time across the two groups. A significance level of p < 0.05 was applied to all statistical tests.
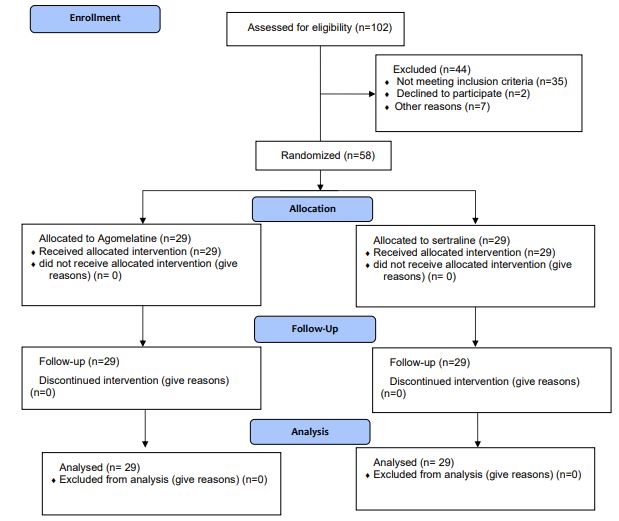
Statistical analysis included data from 29 patients (50.0%) on Sertraline and 29 patients (50.0%) on Agomelatine who completed the study. A 12-week follow-up was considered to evaluate the primary and secondary outcomes. Statistical analysis was performed for all 58 patients. The CONSORT flow chart reports the patient enrollment process (Figure 1).
Most patients in both groups had completed high school (86.2% in the Agomelatine group and 93.1% in the Sertraline group), with no significant difference in education level (p = 0.670). Marital status was similar, with 86.2% of Agomelatine patients and 89.7% of Sertraline patients married (p = 0.687).
Regarding employment, 24.1% of the Agomelatine group were employed compared to 13.8% in the Sertraline group, but this difference was insignificant (p = 0.315). Finally, the NYHA class distribution was similar between the groups, with most patients in classes 2 and 3 showing no significant difference (p = 0.999).
3.2 Primary outcomes
At the beginning of the study, there was no significant difference in the BDI-II questionnaire scores between the groups before the intervention (p = 0.287). Both the Agomelatine and Sertraline groups exhibited a gradual decrease in BDI-II questionnaire scores, with a notable reduction by the twelfth week compared to baseline scores. In the Agomelatine group, the score decreased from 17.18 to 11.66; in the Sertraline group, it decreased from 17.59 to 11.24. However, these reductions were not statistically significant (p > 0.05). The efficacy of Agomelatine compared to Sertraline, based on BDI-II questionnaire scores over time, was similar. Mixed ANOVA demonstrated an overall improvement in depression for patients, regardless of the medication received (p = 0.538) (Table 2).
BDI-II Similarly, at the beginning of the study, the HAM-D score did not differ between the groups before the intervention (p=0.566). In both groups, HAM-D questionnaire scores decreased gradually with a significant reduction by the 12th week, although these reductions weren't statistically significant (p>0.05). Table 2 shows scores decreased from 12.86 to 7.10 in the Agomelatine group and 12.41 to 6.69 in the Sertraline group.
The efficacy of Aomelatine compared to Sertraline, based on the HAM-D questionnaire, was found to be similar. The Mixed ANOVA test showed an overall improvement in depression for patients regardless of the medication received (p=0.412). Significant intra-group differences were observed in the Agomelatine and Sertraline treatment groups at various time points, including before the intervention, the fourth week, the eighth week, and the twelfth week (p<0.001) (Table 2).
Comparing the efficacy of Agomelatine and Sertraline in treating depression in HF patients with NYHA Class I-IV based on the BDI-II questionnaire (All p<0.001) shows a significant and gradual decrease in the score of the BDI-II questionnaire in both treatment groups in HF patients in Class I-IV, compared to before the intervention. These differences were statistically significant (P<0.001). These results indicate that both medications in the BDI-II questionnaire were equally effective in Class I-IV and reduced the patients' depression symptoms (All p<0.001).
Similar findings were observed in the comparison of the efficacy of Agomelatine and Sertraline in treating depression in HF patients in Class I-IV based on the HAM-D questionnaire. Both treatment groups demonstrated a significant reduction in scores over time (P<0.001), as illustrated in Figure 4 (P<0.001). These results affirm the equal effectiveness of both medications in reducing depression symptoms in HF patients in Class I-IV.
3.3 Secondary outcomes
The Chi-square test results, comparing the side effects of medications between the Agomelatine and Sertraline groups, revealed no statistically significant difference (p>0.05). The most common side effect in the Sertraline group was nausea, observed in 7 patients (70%), while the most common side effect in the Agomelatine group was sleep disorders (falling asleep late and waking up early) observed in 6 patients (60%). Overall, the side effect profiles of the two medications did not significantly differ (p>0.05 (Table 3)).
Table 1. Baseline characteristics of randomized participants*
| Characteristic | Agomelatine(N=29) | Sertraline (N=29) | p-value | |
| Age (years) (Mean ± SD) |
61.59±15.32 | 61.38±14.23 | 0.958** | |
| Gender N (%) |
Male | 12(41.40) | 14(48.30) | 0.597 |
| Female | 17(58.60) | 15(51.70) | ||
| Level of Education | High school | 25(86.20) | 27(93.10) | 0.670 |
| College education | 4(13.80) | 2(6.90) | ||
| marital status | married | 25(86.20) | 26(89.70) | 0.687 |
| Single | 4(13.80) | 3(10.30) | ||
| Employment status | employed | 7(24.10) | 4(13.80) | 0.315 |
| Unemployed | 22(75.90) | 25(86.20) | ||
| Etiology | ischemic heart failure | 12(42.90) | 16(57.10) | 0.293 |
| non-ischemic heart failure | 17(56.70) | 13(43.30) | ||
| NYHA class# | 1 | 6(20.70) | 6(20.70) | 0.999 |
| 2 | 10(34.50) | 11(37.90) | ||
| 3 | 10(34.50) | 10(34.50) | ||
| 4 | 3(10.30) | 2(6.90) | ||
| Underlying disease (Thyroid diseases and hyperlipidemia) | Yes | 16(55.20) | 13(44.80) | 0.431 |
| No | 13(44.80) | 16(55.20) | ||
* Mean ± standard deviation or frequency (%), # NYHA: New York Heart Association, **Independent Sample t-test
Table 2. Comparison of Primary Outcomes in Patients Treated with Agomelatine vs. Sertraline at Different Time Points a
| Primary outcomes | Agomelatine (N=29) | Sertraline (N=29) | p-value (BGb) | Mean differenced | ||
| BDI-II questionnaire | ||||||
| Before intervention | 18.17±2.18 | 17.59±1.95 | 0.287 | -0.58(-1.67 to 0.50) | ||
| Week 4 | 16.03±1.95 | 15.69±1.92 | 0.502 | -0.34(-1.36 to 0.67) | ||
| p-value (WGc) | <0.001 | <0.001 | ||||
| Week 8 | 13.79±2.16 | 13.52±2.29 | 0.639 | -0.27(-1.44 to 0.89) | ||
| p-value (WGc) | <0.001 | <0.001 | ||||
| Week 12 | 11.66±2.67 | 11.24±2.70 | 0.561 | -0.41(-1.83 to 1.00) | ||
| p-value (WGc) | <0.001 | <0.001 | ||||
| p-valuee (Weeks 4,8,12) | 0.538 | |||||
| HAM-D questionnaire | ||||||
| Before intervention | 12.86±2.60 | 12.41±3.26 | 0.566 | -0.44(-2.00 to 1.10) | ||
| Week 4 | 11.07±1.73 | 10.66±2.84 | 0.507 | -0.41(-1.65 to 0.82) | ||
| p-value (WGc) | <0.001 | <0.001 | ||||
| Week 8 | 8.93±1.28 | 8.62±2.17 | 0.512 | -0.31(-1.25 to 0.62) | ||
| p-value (WGc) | <0.001 | <0.001 | ||||
| Week 12 | 7.10±1.37 | 6.69±1.94 | 0.354 | -0.41(-1.30 to 0.47) | ||
| p-value (WGc) | <0.001 | <0.001 | ||||
| p-valuee (Weeks 4,8,12) | 0.412 | |||||
a mean ± standard deviation, b Between group analysis, c Within group analysis, d Mean (95% confidence interval), e Mixed ANOVA
Table 3. Comparison of Secondary Outcomes in Agomelatine and Sertraline Treatment Groups a
| Secondary outcomes N (%) |
Agomelatine (N=29) | Sertraline (N=29) | p-value |
| Sleep Disorder | 6 (20.70) | 4 (13.80) | 0.487 |
| Sexual Disorder | 1 (3.40) | 1 (3.40) | 0.999 |
| Weight gain | 1 (3.40) | 2 (6.90) | 0.999 |
| Weight Loss | 1 (3.40) | 1 (3.40) | 0.999 |
| Nausea | 3 (10.30) | 7 (24.10) | 0.164 |
| Diarrhea | 2 (6.90) | 5 (17.20) | 0.423 |
| Constipation | 1 (3.40) | 2 (6.90) | 0.999 |
| Headache | 4 (13.80) | 6 (20.70) | 0.487 |
| Tremor | 1 (3.40) | 2 (6.90) | 0.999 |
| Hyperhidrosis | 0 (0.0) | 3 (10.30) | 0.237 |
| Decrease Saliva | 0 (0.0) | 2 (6.90) | 0.491 |
| Somnolence | 4 (13.80) | 3 (10.30) | 0.999 |
| Palpitation | 0 (0.0) | 1 (3.40) | 0.999 |
a Chi-Square & Fisher’s Exact Test
Discussion
The result of this study, comparing the efficacy of Agomelatine and Sertraline in treating depression symptoms in HF patients over the twelve-week follow-up, showed a reduction in depression symptoms in both treatment groups during twelve weeks based on the BDI-II and HAM-D. Both Agomelatine and Sertraline were effective, with no significant difference observed between the two medications.
HF patients with depression need to be treated immediately, as depression worsens their prognosis. As a result of non-adherence to medication, depression can lead to heart failure and reduce the possibility of successful disease management (22). Similarly, Fadhil et al. reported improvement in depressive symptoms in chronic kidney disease (CKD) patients with Agomelatine and Sertraline treatment (23). Moreover, Medvedev et al. examined the effects of Agomelatine on depression in patients with underlying cardiovascular disease. They found that Agomelatine significantly improved depression and anxiety symptoms in cardiovascular patients with depression. According to a previous study (24), Agomelatine is safe for the treatment of depression in cardiovascular patients. Treatment for depression should not only alleviate symptoms, but also promote normal functioning and prevent recurrence of the illness (25). The present study confirms the findings of a meta-analysis study demonstrating Agomelatine's effectiveness as an alternative to standard antidepressants. Therefore, Agomelatine has similar properties as other antidepressants. The observed results showed no significant differences in effectiveness between Agomelatine and Sertraline, both considered SSRI medications (26). Based on Cipriani et al.'s meta-analysis, Agomelatine was found to be more effective and acceptable than other antidepressants for treating depression in adults. Our study found Agomelatine's efficacy similar to Sertraline, a proven antidepressant. Discrepancies might arise from comparing Agomelatine to one medication or comparing meta-analyses to clinical trials (27). The side effects of Sertraline and Agomelatine were similar in a study performed in Bangkok in 2022 (23). Based on a systematic review and meta-analysis by Oliva et al, it appears that Sertraline is associated with more gastrointestinal side effects compared with other antidepressants, including Mirtazapine (28).
Clinical studies have demonstrated Agomelatine's antidepressant efficacy. In a large-scale clinical trial involving individuals with major depressive disorder, Agomelatine exhibited good tolerability along with its antidepressant activity. Some of the side effects of Agomelatine include rare reports of sexual discomfort, and it appears to be safe if taken in excess. Agomelatine can be a valuable additional treatment option for patients who do not fully respond to treatment or cannot tolerate the side effects of other antidepressants. The reason is that Agomelatine has a different action mechanism than other medications (29). However, these findings are not entirely consistent with our study, possibly because the referenced study did not compare the Agomelatine group with another medication group, focusing solely on Agomelatine's effects.
In another study focused on the effect of Agomelatine and Sertraline for the treatment of depression in type 2 diabetes, they concluded that Agomelatine may help treat depression and anxiety symptoms, with potential advantages over Sertraline. It was noted that Agomelatine might enhance health-related behaviors in depressed type 2 diabetes patients. These two antidepressants were well tolerated, and none of the patients left the study (30). Despite differences in results, potential factors contributing to this inconsistency include variations in study populations, the relatively small sample size, and the absence of a placebo group. However, both studies concurred on the side effects of the two medications.
One study showed that Agomelatine was more effective than Sertraline in treating major depressive disorder, and this is not consistent with the results of our study (31). Conversely, a study assessing the safety and efficacy of Sertraline and Agomelatine in hemodialysis patients reported satisfactory antidepressant effects with Sertraline but no improvement with Agomelatine. This inconsistency could stem from differences in the study population. These findings are not similar to our study (32). Overall, the similarity in the effects of Agomelatine and Sertraline in reducing depression symptoms may be due to their similar mechanisms in increasing serotonin levels and improving patients' clinical status. However, there may be minor differences in side effects, long-term effects, or the precise mechanisms of action of each drug, which should be explored in future studies.
Strengths and limitations of the study
Double-blindness, standardized tools for depression assessment, and a focus on HF patients with depression symptoms are some of the strengths of our study. Methodology was one of the study's strengths, which included follow-ups and evaluations every four, eight, and twelve weeks. Through this approach, it was possible to monitor changes and gauge treatment effects over time. Furthermore, comparing the efficacy of two different medications, Sertraline and Agomelatine, for patients with HF provided valuable insight into their treatment options. As a result, the study is valid and has scientific value, furthering medical knowledge and our understanding of treatments for depression in patients with heart failure.
There are, however, several limitations to this study. It is possible that the findings cannot be generalized due to the small sample size. Patients with HF may benefit from a more extensive and diverse population study to gain more insight into Agomelatine's and Sertraline's relative effectiveness in treating depression. Results may not be captured in the twelve-week follow-up period as long-term treatment effects and durability may not be captured. It is important to follow up on both medications to determine sustained efficacy and potential relapse rates. Due to the absence of a placebo control group, it is difficult to attribute observed improvements solely to medication effects.
Recommendation for future research
A more extended follow-up period, such as six months or one year, would provide a comprehensive assessment of treatment effectiveness and potential relapse rates in future studies. A placebo group should be included in future research so that placebo effects can be examined and specific medication effects can be isolated. Increasing the size and diversity of the research population, including different demographic characteristics and levels of heart failure severity, would enhance external validity. Long-term side effects and tolerability of Agomelatine and Sertraline could provide crucial insights into their sustainability. Future research could examine these medications' effects on heart failure patients' hearts.
Conclusion
Hysteroscopic myomectomy for submucosal myoma in infertile patients is a safe procedure without major complications.
Declarations
Acknowledgements
We would like to express our sincere gratitude to everyone who contributed to this study. We are especially thankful to Babol University of Medical Sciences for their financial support.
Ethical Considerations
Under the Ethics Committee of Babol University of Medical Sciences, the study was approved (Approval ID: IR.MUBABOL.REC.141138) and registered in the Iranian Registry of Clinical Trials (Registration Number: IRCT20170606034348N7). All participants signed an informed consent form following detailed explanations of the study's objectives and procedures.
Authors' Contributions
N.Z. was involved in data collection, data analysis, manuscript writing, and editing; A.H., A.R., S.A., H.SH, H.H., and N.T. contributed to project development, data analysis, and editing; and R.H. participated in project development, data analysis, manuscript writing, and editing. All authors approved the submitted version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest regarding the publication of this study.
Fund or Financial Support
We sincerely thank Babol University of Medical Sciences for their financial support.
Using Artificial Intelligence Tools (AI Tools)
The authors were not utilized AI Tools.
Received: 2024/11/21 | Accepted: 2025/03/3 | Published: 2025/03/13
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |