BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://journal.zums.ac.ir/article-1-7574-en.html
2- Department of Neurology, School of Medicine, Zanjan University of Medical Sciences, Zanjan, Iran & Stroke Research Group, Valiasr Hospital, Zanjan University of Medical Sciences, Zanjan, Iran ,
Migraine is a genetically influenced and complex neurological disorder manifesting as recurring episodes of moderate-to-severe headaches. Migraines, a common form of headache disorder, are characterized by unilateral pain often accompanied by debilitating symptoms such as nausea, sensitivity to light (photophobia), and sensitivity to sound (phonophobia) (1). Headache disorders, including migraines, represent a major global public health challenge due to their high prevalence as well as profound societal and individual impacts. Approximately one billion people worldwide are estimated to suffer from headache disorders, highlighting the widespread nature of this condition (2). Migraines, in particular, have been identified as a leading cause of disability. In 2019, they were ranked as the second most common cause of disability globally and the most frequent cause among women under the age of 50 (3). In the United States, migraines result in an estimated $13 billion in annual productivity losses, with total costs potentially reaching $78 billion when including medical expenses and reduced quality of life (2).
Given the considerable toll migraines take on an individual's quality of life, it is crucial that effective treatment strategies not only target the primary symptoms of migraines, such as headache pain, nausea, and sensitivity to light and sound, but also consider any coexisting or comorbid conditions the patient may have, such as anxiety, depression, or sleep disturbances. Additionally, treatment plans should be tailored to align with the patient’s expectations, personal needs, and long-term health goals to ensure better adherence and overall satisfaction with the therapeutic approach (4).
Currently, treatment options for migraines encompass both pharmacological and non-pharmacological approaches, each varying in its effectiveness for providing pain relief and improving a patient's functional ability. Pharmacological treatments typically include acute medications to relieve symptoms during an attack as well as preventive medications aimed at reducing the frequency and severity of future episodes. Non-pharmacological methods may involve lifestyle changes, stress management techniques, cognitive-behavioral therapy, and other holistic or integrative health practices (5-8). However, many anti-migraine medications available today do not provide complete relief and are often accompanied by various undesirable side effects, such as dizziness, fatigue, gastrointestinal disturbances, or even medication overuse headaches. These side effects can significantly impact the patient's quality of life, potentially reducing adherence to the treatment regimen (9, 10). Thus, there is a pressing need for the development of new therapies that can offer equal or superior clinical effectiveness while minimizing adverse effects, thereby providing a more balanced and tolerable approach to managing migraines.
One emerging alternative is the use of devices that apply magnetic stimulation or electrical current to the head or body. These devices have been approved by the US Food and Drug Administration for migraine treatment, with clinical trials showing improvements in pain relief for acute migraines and reductions in migraine days by up to two per month (3). In our population study, we focused on the use of Cefaly® as a non-pharmacological method, particularly for individuals who prefer to avoid prolonged pharmacological treatments due to cultural reasons or concerns about adverse reactions.
Peripheral nerve stimulation, particularly via an external trigeminal nerve stimulation (e-TNS) device such as Cefaly®, has shown promising results in treating chronic and episodic migraines (11). The Cefaly® device stimulates both the supratrochlear and supraorbital nerves-branches of the trigeminal nerve—bilaterally using a self-adhesive bipolar electrode applied to the forehead (12). The e-TNS method, such as the use of devices like Cefaly®, is recognized for its strong safety profile and high tolerability, making it particularly beneficial for patients who cannot use standard migraine medications such as triptans or who suffer from adverse effects when taking nonsteroidal anti-inflammatory drugs (NSAIDs). Triptans, which are commonly prescribed for acute migraine relief, are not suitable for everyone due to potential side effects including chest pain, flushing, or cardiovascular complications. Similarly, NSAIDs, while effective for many, can cause gastrointestinal discomfort, ulcers, and other issues, especially in those with sensitivities or when used frequently (13).
A single application of e-TNS has been found to relieve migraine pain without affecting cerebral metabolism (14). Meanwhile, long-term use, as observed in FDG-PET studies, can increase metabolism and significantly lower the frequency of migraine attacks after several months of daily application (15). Given these metabolic changes, patients experience a significant reduction in attack frequency (12).
Transcutaneous supraorbital neurostimulation (tSNS), a specific application of e-TNS, has demonstrated efficacy in preventing episodic migraines. The PREMICE trial, a randomized double-blind sham-controlled study, utilized the Cefaly® device to stimulate both supraorbital nerves. Over a 3-month period, active tSNS revealed a 50% response rate of 38.2%, with significant effects comparable to other migraine prevention therapies, and no adverse effects or dropouts related to the device (13).
Given the crucial role of trigeminal nerve stimulation in the treatment of migraines, this study aims to explore the specific effects of nerve stimulation using the Cefaly® device. The primary goal of this research is to evaluate whether the use of Cefaly®, in conjunction with standard migraine medications, provides an effective strategy for mitigating the symptoms of migraines, including pain severity, nausea, as well as sensitivity to light and sound. By combining the device's non-invasive nerve stimulation with pharmacological treatments, the study seeks to determine if there is a synergistic effect that would enhance patient outcomes beyond what is achieved by medications alone. Additionally, the study focuses on assessing the effectiveness of targeted stimulation of the supraorbital and trigeminal nerves in reducing the overall intensity, frequency, and duration of migraine attacks across a specific patient population in western Iran. The research will explore whether this combined approach can offer significant improvements in managing migraines, potentially providing a more comprehensive and tailored treatment strategy. The findings of this study could help in better understanding the potential benefits of integrating e-TNS with conventional therapies, ultimately leading to improved quality of life for migraine sufferers.
A monitoring form was developed to track the progress of participants before and after the intervention. A questionnaire was designed to assess the severity, duration, and frequency of migraine symptoms as defined by the IHS migraine diagnostic criteria. Each patient was monitored over the course of one month, with their attack frequency, severity, and duration being recorded. Due to the limited availability of devices, the study extended over approximately ten months in total. The intensity of pain, duration of attacks, and number of attacks were classified according to the pretreatment migraine headache questionnaire used at Massachusetts General Hospital (17). Pain intensity was categorized into three levels: 1 to 3, 4 to 7, and 8 to 10. The number of attacks was grouped as follows: 1 to 3 attacks, 3 to 5 attacks, and more than 5 attacks. Attack duration was divided into the following subgroups: less than 3 hours, 3 to 5 hours, 5 to 12 hours, 12 to 24 hours, and more than 24 hours. The CEFALY® device (CEFALY Technology, Seraing, Belgium) was used for neurostimulation in this study. This device generates a constant current with a maximum skin impedance of 2.2 kΩ and delivers rectangular biphasic symmetrical pulses with a zero electrical mean. The electrical pulses are transmitted transcutaneously via a supraorbital bipolar self-adhesive electrode placed on the forehead, stimulating the supratrochlear and supraorbital nerves bilaterally (12). In this study, the verum device operated at a pulse frequency of 100 Hz and a pulse width of 250 µs. Proper use required a clean forehead, particularly in the area between the eyebrows where the electrode would be attached, and repeated pressing of the electrode to ensure a secure connection. The effectiveness of the treatment was contingent upon the quality of the electrode-skin connection.
Three programs were designed and implemented to evaluate the device's effectiveness in treatment, prevention, and relaxation. The first program targeted the treatment of migraine attacks. The second program was intended for the prevention of migraine attacks. The third program aimed to provide relaxation, recreation, and acclimatization to the device (Fig 1). During migraine attacks (with or without aura), the patients were instructed to use the first program, and indeed in this study, we only used the first program. The patients were permitted to use the device for a maximum of 20 minutes per session. The study was approved by the ethics committee of the participating organization. All participants provided written informed consent. Confidentiality of patient information was strictly maintained.
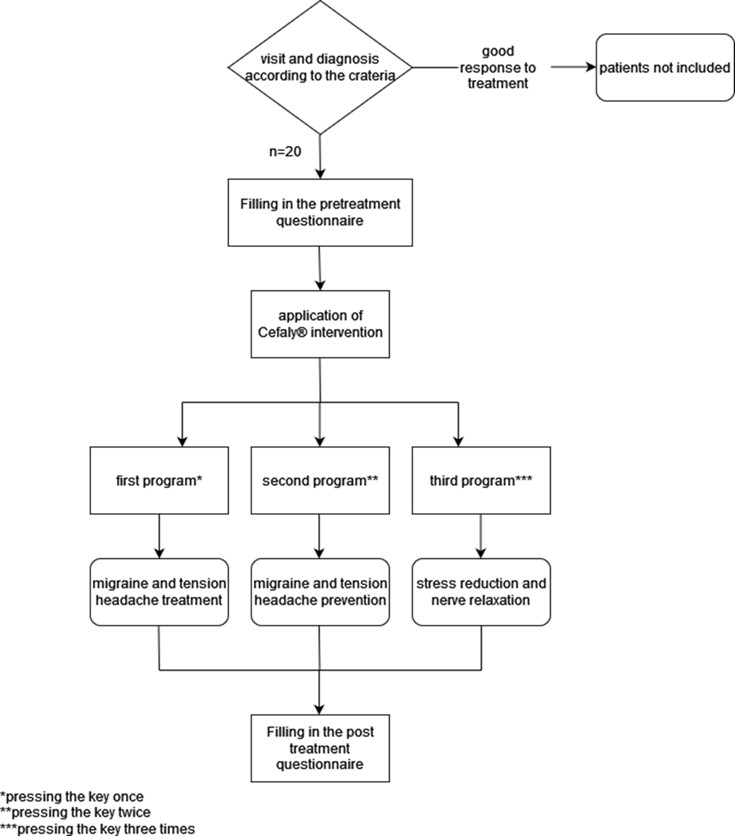
Figure 1. Study design flowchart of the participants.
Table 1. Frequency of participants before and after intervention.
| Variable | Categories | N |
| Sex | Female | 7(35%) |
| Male | 13(65%) | |
| Marital Status | Married | 15(75%) |
| Single | 5(25%) | |
| Drug Treatment | Sumatriptan or Ergotamine | 10(50%) |
| Sedative | 10(50%) | |
| Duration of migraine | 5 years< | 11(55%) |
| 5-10 years | 7(35%) | |
| 10 years> | 2(10%) | |
| Duration of attack | 3 hours< | 0(0%) |
| 3-5 hours | 1(5%) | |
| 5-12 hours | 3(15%) | |
| 12-24 hours | 7(35%) | |
| 24 hours> | 9(45%) | |
| Number of attacks | 1-3 | 1(5%) |
| 3-5 | 15(75%) | |
| 5> | 4(20%) | |
| Migraine with aura | Yes | 13(65%) |
| No | 7(35%) | |
| Severity | Mild | 3(15%) |
| Moderate | 8(40%) | |
| Severe | 9(45%) |
| Variable | Categories | Before intervention n (%) | After intervention n (%) | P value | ||
| Severity | Mild | 3(15%) | 11(55%) | 0.037 | ||
| Moderate | 8(40%) | 6(30%) | ||||
| Severe | 9(45%) | 3(15%) | ||||
| Number of attacks | 1-3 | 1(5%) | 11(55%) | 0.038 | ||
| 3-5 | 15(75%) | 9(45%) | ||||
| 5> | 4(20%) | 0(0%) | ||||
| Duration of attack | 3 hours< | 0(0%) | 0(0%) | 0.002 | ||
| 3-5 hours | 1(5%) | 5(25%) | ||||
| 5-12 hours | 3(15%) | 8(40%) | ||||
| 12-24 hours | 7(35%) | 4(20%) | ||||
| 24 hours> | 9(45%) | 3(15%) | ||||
| Severity | Mild | 3(15%) | 11(55%) | 0.037 | ||
| Moderate | 8(40%) | 6(30%) | ||||
| Severe | 9(45%) | 3(15%) | ||||
| Number of attacks | 1-3 | 1(5%) | 11(55%) | 0.038 | ||
| 3-5 | 15(75%) | 9(45%) | ||||
| 5> | 4(20%) | 0(0%) | ||||
| Duration of attack | 3 hours< | 0(0%) | 0(0%) | 0.002 | ||
| 3-5 hours | 1(5%) | 5(25%) | ||||
| 5-12 hours | 3(15%) | 8(40%) | ||||
| 12-24 hours | 7(35%) | 4(20%) | ||||
| 24 hours> | 9(45%) | 3(15%) | ||||
Discussion
This prospective study evaluated the effects of trigeminal nerve subcutaneous stimulation (e-TNS) on the intensity, frequency, and duration of migraine attacks in 20 patients who had not adequately responded to standard treatments. The analgesic effects of trigeminal nerve stimulation can be primarily understood through the gate control theory of pain, which explains how pain modulation occurs in the nervous system. According to this theory, the activation of large afferent sensory fibers-such as those targeted during trigeminal nerve stimulation—can inhibit or "gate" the transmission of pain signals that are carried by smaller nerve fibers to the brain. By stimulating the larger sensory fibers, the nerve stimulation essentially reduces the transmission of pain signals, raising the pain threshold and diminishing the patient's perception of pain (18). In addition to the gate control theory, evidence from both animal studies and neuroimaging research in humans suggests that trigeminal nerve stimulation may activate central pain inhibitory systems within the brain. These central mechanisms are believed to involve areas such as the periaqueductal gray matter, the rostral ventromedial medulla, as well as other parts of the brainstem that play a critical role in modulating pain perception. By engaging these central pain inhibitory pathways, trigeminal nerve stimulation could help further suppress pain signals and contribute to its overall effectiveness in mitigating migraine symptoms.
Our study demonstrated that the Cefaly® device could effectively reduce migraine severity in patients unresponsive to conventional treatments, with no reported adverse effects. Following the intervention, significant reductions were observed in the intensity, frequency, and duration of migraine attacks, underscoring the device's clinical efficacy. Therefore, Cefaly® can be used as a maintenance treatment alongside pharmacotherapy.
Chou et al (19) reported that Cefaly® effectively reduced headache intensity, consistent with our findings of a decline in severe migraine attacks. This reduction in severity was clinically significant. Similarly, Russo et al (20) found that migraine attacks dropped by at least 50% in clinical trials involving similar interventions. Schoenen et al (13) noted that while pharmacological treatments for migraines can be effective, they often come with various side effects. In contrast, e-TNS offers advantages such as better tolerance and safety (13). Cefaly® also presents fewer contraindications compared to traditional anti-migraine drugs (21). Despite the small sample size, three neurostimulation programs were evaluated for their effects on treatment, prevention, and relaxation. The intervention’s effectiveness was assessed based on changes in attack frequency and severity.
The study faced several limitations that should be considered when interpreting its results. One of the primary limitations is insufficient funding, which may have restricted the scope and scale of the research, including the ability to recruit a larger and more diverse patient population. As a result, the study was limited to a relatively small sample size, consisting only of patients who had not responded to conventional migraine treatments. These limitations may affect the generalizability of the findings. However, the data can inform future clinical trials. Despite the limited sample size and study duration, all participants completed follow-up, and no patients were lost to follow-up. The main weaknesses of the study were its low statistical power and limited generalizability. However, similar findings in other studies suggest that larger future studies could provide more comprehensive insights.
Future research with larger sample sizes could more accurately measure various factors, including the impact of the prophylactic potential of the Cefaly® device, and other detailed aspects. Renting devices could lower research costs. Additionally, precise assessments and further studies could enhance understanding of quality-of-life improvements achieved with the Cefaly® device. A cost-benefit analysis comparing e-TNS with other treatments for migraines could provide valuable information on its relative economic and therapeutic value.
Conclusion
This study provided compelling evidence that trigeminal nerve stimulation, specifically using the Cefaly® device, is an effective method for reducing the severity, frequency, and duration of migraine attacks in patients who have not responded to conventional treatments. The findings demonstrated a significant reduction in the intensity of migraine episodes, with a notable reduction in the proportion of participants experiencing severe attacks. Additionally, the data showed a substantial reduction in the number of migraine occurrences per month, indicating that regular use of the Cefaly® device can help lower the overall frequency of migraine episodes for these patients. Further, the study also highlighted a meaningful decline in the duration of individual migraine attacks, suggesting that the Cefaly® device not only makes migraines less frequent but also less prolonged. The observed improvements in migraine outcomes underscore the efficacy of trigeminal nerve stimulation in alleviating migraine symptoms and enhancing patient quality of life. These results support the use of Cefaly® as a viable non-pharmacological adjunct for patients seeking relief from chronic and severe migraines. Further research with larger sample sizes and longer follow-up periods is recommended to validate these findings and explore the long-term benefits of this intervention.
Declarations
Acknowledgements
In particular, we highly appreciate the doctors and nurses in the neurology ward at Vali-Asr Hospital for saving lives, along with Dr.Masoud Asgari for providing technical help and writing assistance.
Ethical Considerations
Ethical approval for this study was obtained from the ethics committee (IR.ZUMS.REC.1398.399), and all patients provided informed consent for the use of their data in clinical research. The study adhered to the principles outlined in the latest version of the Declaration of Helsinki.
Authors' Contributions
Writing-original draft preparation, M.A. and P.K. and T.E.; funding acquisition, A.G.; Writing-review and editing, A.G. The final manuscript was read and approved by all of the authors.
Conflicts of Interest
The authors declareThe authors declare that there are no conflicts of interest.
Fund or Financial Support
This research received no specific grant from any funding agency in the public, commercial, or not for profit sector.
Using Artificial Intelligence Tools (AI Tools)
The authors were not utilized AI Tools.
Received: 2024/12/2 | Accepted: 2025/03/5 | Published: 2025/03/13
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |







