BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://journal.zums.ac.ir/article-1-6895-en.html


 , Gholamhosein Meftahi2
, Gholamhosein Meftahi2 

 , Farideh Bahrami3
, Farideh Bahrami3 

 , Zahra Bahari3
, Zahra Bahari3 

 , Ali Zarei Mahmoudabadi1
, Ali Zarei Mahmoudabadi1 

 , Hasan Fallah Huseini4
, Hasan Fallah Huseini4 

 , Zohreh Jangravi *5
, Zohreh Jangravi *5 


2- Neuroscience Research Center, Baqiyatallah University of Medical Sciences, Tehran, Iran
3- Dept. of Physiology and Medical Physics, Faculty of Medicine, Baqiyatallah University of Medical Sciences, Tehran, Iran
4- Medicinal Plants Research Center, Institute of Medicinal Plants, ACECR, Karaj, Iran
5- Dept. of Biochemistry, Faculty of Medicine, Baqiyatallah University of Medical Sciences, Tehran, Iran ,
✅ Ery extract, as a safe herbal extract, has anti-adipogenic activity and merits more investigation as a candidate for developing an anti-obesity drug or supplement.
Obesity is a major global health and economic challenge due to its direct association with serious conditions such as type 2 diabetes mellitus, cancer, coronary heart disease, and sleep apnoea (1,2). Considering changes in food consumption patterns and inactive lifestyle, the incidence of obesity is increasing more rapidly worldwide (3). The treatment and control of obesity are major priorities to reduce financial burdens and the burden of chronic diseases (4).
Although dietary and lifestyle control has been considered the leading method to combat obesity, pharmacological and surgical treatment are unavoidable in most cases (5,6). Nevertheless, severe side effects, especially in the long-term application of synthetic compounds, weight loss efficacy, surgical risks, and obesity relapse are the main limitations of current therapies (7). Due to lower side effects and greater cost-effectiveness, natural products have attracted research attention for the development of anti-obesity agents or supplements (6).
At tissue and cellular levels, obesity is defined as hypertrophy and hyperplasia of adipocytes which increases the adipose tissue mass (4). Hypertrophy means the expansion of adipocytes, which is associated with accumulated fat as triglycerides (TGs) in adipocytes. Hyperplasia or adipogenesis has been considered a complex process of pre-adipocytes' proliferation and differentiation which eventually determines the number of adipocytes and is characterized by the accumulation of membrane-bound lipid droplets (8). Therefore, the regulation of adipogenesis, which determines the size and number of adipocytes, can be a possible therapeutic approach to obesity (4).
Eryngium is a flowering plant belonging to the Apiaceae family which comprises about 230–250 species. Many Eryngium species have been used as food and medicine. Flavonoids, phytosterols, triterpenoid saponins, ecdysteroids, coumarin derivatives, polyacetylenes, phenolic acids, and essential oils are the main phytonutrient components of Eryngium (9,10).
E. billardierei is a well-known medicinal plant with anti-inflammatory and anti-hyperglycemic effects. It has been widely recommended for the treatment of diabetes. Various parts of this plant are used for treating scorpion bites, rheumatism, urinary infections, wound healing, sinusitis, goiter, etc. The phytochemical component is extracted from the root and aerial parts of this plant (11-13).
In this study, the 3T3-L1 murine preadipocyte cell line, as a commonly used cell line model for in vitro study of adipogenesis, was differentiated to adipocyte to evaluate the anti-adipogenic effect of the alcoholic extract of E. billardierei F. Delaroche extract.
Plant material
This study was approved by the Ethics Committee of Baqiyatallah University of Medical Sciences, Tehran, Iran (IR.BMSU.REC.1399.234).
E. billardierei specimens were collected from the Alamoot region of Qazvin Province (Iran) on May 2019, and the plant identity was verified by a botanist (M. Ahvazi). A voucher specimen of the plant was pressed and kept at the Central Herbarium of the Research Institute of Medicinal Plants, ACECR Alborz, Iran (761(IMPH)). E. billardierei specimens were washed, dried, and crushed for extraction.
Extract Preparation
The extract of dried aerial parts of E. billardierei was prepared with methanol using the percolation method (14). Briefly, 500 g of chopped material was moistened with a sufficient amount of methanol and placed in a suitable percolator; the solvent was added to completely saturate the wet mass. After 24 hours, the valve of the percolator was opened, and the plant extract came out drop by drop from the end of the percolator. The extraction process was repeated two more times with the addition of fresh solvent. The extract was filtered by filter paper (25 mm in diameter, Whatman No. 1) and evaporated to dryness under reduced pressure at a maximum of 40 ºC using a rotary evaporator instrument (Heidolph, Germany).
Cytotoxicity Assay
The MTT assay was used to measure the cytotoxic concentration of E. billardierei extract against 3T3-L1 cells. The cells were treated with different concentrations of E. billardierei extract at 0.5, 1.5, 3, 4.5, 6, 8, and 10 mg/ml for 96 h. Then, the final concentration of 0.5 mg/ml of the MTT solution in phosphate-buffered saline (PBS) was added to each well and incubated for 4 hours. After that, the MTT solution was removed, 150 ml of the DMSO solution was added, and the absorbance was read at 590 nm.
Cell Culture and Differentiation of Adipocytes
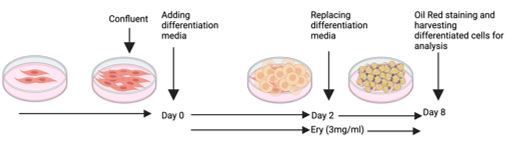
The 3T3-L1 fibroblast cell line was purchased from the National Cell Bank of the Pasteur Institute of Iran. The cells were cultured in a complete medium containing Dulbecco’s modified eagle medium (DMEM, Bio-IDEA) supplemented with 10% fetal bovine serum (FBS, Bio-IDEA) and 1% penicillin/streptomycin (P/S, Bio-IDEA) at 37 °C in a CO2 incubator. Two days after confluency (designated as day 0), the differentiation medium containing 0.25 μM dexamethasone (DEX, Iran Hormone), 0.5 mM 3-isobutyl-1-methylxanthine (IBMX, Sigma-Aldrich), and 1 μg/mL insulin (Novo Nordisk) was added to the culture medium to stimulate differentiation. After 48 h, the medium was refreshed with DMEM supplemented with 10% FBS every 48 h. The E. billardierei extract was added to the culture medium from day 0 to day 8 (Figure 1). The experimental groups included control (without differentiation and extract treatment), Diff (in which cells were differentiated without extract treatment), and Diff+Ery (in which cells were differentiated and treated with E. billardierei extract as described above).
Figure 1. Schematic representation of preadipocyte differentiation into mature adipocyte. The procedure has been completely described in material and method section.
Oil Red O Staining
Adipocyte lipid accumulation was investigated by Oil Red O (ORO, Bio-IDEA) staining assay. On day 8, in every group, cells were washed with PBS and fixed with 3.7% (v/v) formaldehyde for 1 h. Cell staining was performed for 30 min with 60% isopropanol in filtered ORO solution (6:4 of Oil Red stock solution to distilled water). After washing the excess stain with distilled water three times, lipid droplets were observed under an inverted light microscope.
Triacylglycerol assay
On day 8, cells were washed gently with PBS and lysed with PBS supplemented with 1% TritonX-100, and the sonicated triacylglycerol (TG) content was estimated by colorimetric methods using commercially available kits (Pars Azmoon Laboratories). Triacylglycerol was normalized to protein content determined by the Bradford method.
GPDH activity assay
The activity of the GPDH enzyme was estimated using a GPDH activity assay kit (Sciencell, USA) based on the manufacturer’s instructions. Briefly, the cells were scraped from the plate into a homogenization buffer and centrifuged at 10,000 × g for 10 minutes at 4 oC to remove the insoluble material. A decrease in NADH absorbance at 340 nm was used to determine the GPDH activity. Protein concentration was measured by the Bradford method.
Statistical analyses
Values are presented as the mean ± standard deviation (SD). One-way ANOVA was used for statistical analyses, followed by Tukey's test. All the analyses were performed in SPSS 21.
Nontoxic concentration of E. billardieri
The nontoxic concentration was determined by the MTT assay. Treatment of 3T3-L1 at concentrations of ³4.5 mg/ml of the extract significantly reduced cell viability (P£0.05). No significant reduction was observed in cell viability at concentrations of £3 mg/ml (P>0.05) (Figure 2). Thus, the 3 mg/ml concentration was selected as the maximum nontoxic concertation of the extract for the other assays.
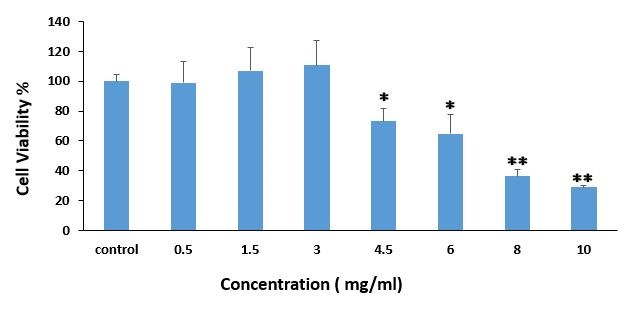
Figure 2. MTT assay used for cell viability determination (for 96h) in the presence of different concentration of Ery extract (0.5–10 mg/ml). Values have been presented as Mean ±SD of of four independent experiments. One- and two-star signs indicate p<0.0.5 and p<0.01 respectively in compare to control group.
E. billardierei treatment inhibited lipid droplet accumulation in 3T3-L1-derived adipocytes
The impact of E. billardierei extract on the accumulation of lipid droplets during differentiation of 3T3-L1 preadipocyte was studied by Oil Red O staining on day 8. As shown in Figure 3, lipid droplets markedly increased during the adipocyte differentiation, and treatment with 3 μg/ml of the extract markedly reduced intracellular lipid droplets' accumulation. The results showed that the E. billardierei extract can suppress adipocytes' differentiation and lipid droplets' accumulation.
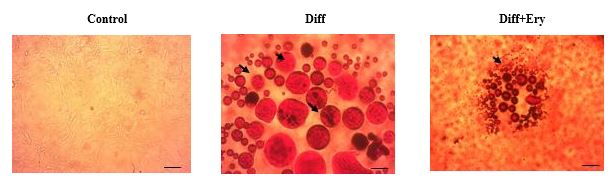
Figure 3. Effect of Ery extract on lipid accumulation in 3T3-L1 adipocyte. Were induced to differentiation of 3T3-L1 preadipocytes into mature adipocytes was stimulated in the presence of and absence of Ery extract. Lipid droplet accumulation was obseved using Oil Red O staining of cells on day 8. Control: without differentiation, Diff: Differentiation without treatment, Diff+Ery: Differentiation with Ery treatment (3mg/ml). Staining has been presented with a 40× magnification and a scale bar 500 μm. Lipid droplets have been pointed by black arrows.
E. billardierei treatment decreased the TG content of 3T3-L1-derived adipocytes
Incubation with the adipocyte hormonal cocktail significantly increased the TG content in the differentiation group (0.78±0.05 mg/mg protein) compared to the control group (0.2±0.04 mg/mg protein) (P<0.01) on day 8. E. billardierei treatment significantly decreased the TG level in the treated group (0.32±0.03 mg/mg protein) compared to the differentiation group (P<0.01) (Figure 4).
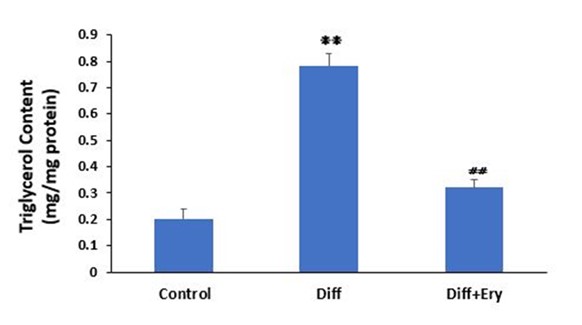
Figure 4. Effect of Ery extract on triglycerol content in 3T3-L1 adipocytes was evaluated by colorimetric assay in each group on day 8. Diff indicates differentiated group without treatment of Ery extract and Diff+Ery indicates differentiated group which received Ery extract for 8days during differentiation procedure. ❋❋ indicates significant change compare to control group (P<0.01) and ⧣⧣ showes significant change compare to Diff group (P<0.01). Values have been presented as Mean ±SD (n=3).
E. billardierei treatment decreased the GPDH activity of 3T3-L1-derived adipocytes
A key indicator of adipocyte differentiation and lipid droplet accumulation is GPDH activity measurement. The GPDH activity assay on day 8 showed that treatment of the E. billardierei extract at a concentration of 3 g/mL induced a significant decrease in GPDH activity (15.3±2.1 U/mg protein) relative to the Diff group (30.2±3.9 U/mg protein) (Figure 5).
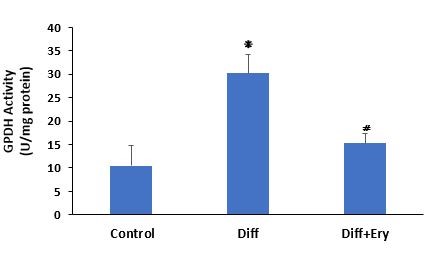
Figure 5. Commercial GPDH activity assay kit wase used for measurement of GPDH activity in each group on day 8. Diff indicates differentiated group without treatment of Ery extract and Diff+Ery indicates differentiated group which received Ery extract for 8 days during differentiation procedure. ❋ indicates significant change compare to control group (P<0.05) and ⧣ shows significant reduction compare to Diff group P<0.01). Values have been presented as Mean ±SD (n=3).
Discussion
Obesity is a chronic and degenerative disease and one of the most common metabolic diseases (3). It has been considered an urgent public health challenge because of its direct association with other diseases (3). Given the side effects and low efficacy of current therapies, natural products have been considered for developing new drugs (6).
In the present study, the effects of E. billardierei alcoholic extract on the adipogenesis of preadipocyte (3T3-L1, mouse-derived fat progenitor cells) were investigated.
3T3-L1 mouse fat progenitor cells are a common cell model in the study of obesity and adipogenesis. In this cell model, fat and triglyceride accumulation can be easily measured in the cell culture medium (15).
Numerous studies have evaluated the effect of different plant extracts on reducing the occurrence of obesity (6,16,17). Rho et al. examined the anti-obesity effects of 400 crude extracts of medicinal plants. Of all the tested plants, they reported 44 extracts with anti-obesity activity (16).
The beneficial properties of Eryngium spp. to treat diabetes, dyslipidemia, hypertension, asthma, burns, fevers, malaria, and digestive problems have been reported in different studies. Flavonoids, tannins, saponins, triterpenoids, and essential oils are the major phytonutrient components of the genus Eryngium spp. The effects of each component have been described (18,19). It has been proved that saponins reduce the serum levels of lipids and total cholesterol and can thus control dyslipidemia (18,19). The results of this study also showed that E. billardierei extract treatment could significantly reduce the formation of lipid droplets, and triglyceride levels increased during 3T3-L1 differentiation. The formation of lipid droplets and triglyceride levels indicate the degree of adipogenesis, and reduced fat droplet formation during 3T3-L1 differentiation can be considered a remarkable feature of plant extracts with anti-adipogenic effects (20-22). Khani et al. showed that the saponin component of E. billardierei extract can change the lipid profile by increasing the biliary secretion of cholesterol and reducing luminal absorption in a diabetic rat model (23). Other studies also found that the ethanolic extract of E. carlinae can diminish total cholesterol and triglyceride levels in Wistar rats with experimental diabetes (24,25).
Glycerol 3-phosphate dehydrogenase (GPDH) as a lipogenic enzyme is another marker in assessing the differentiation of adipocytes (26). The present study showed that in the differential group (Diff), compared to the non-differential group (control), the activity of this enzyme significantly increased at the end of the 8th day of differentiation. E. billardierei reduced the activity of this enzyme in the treatment group which indicates the anti-adipogenic properties of this extract. So far, no study has reported the effect of E. billardierei extract on the differentiation of 3T3-L1 progenitor cells. However, in vitro anti-adipogenic effects of E. billardierei extract have been reported in some studies, which have confirmed the role of this extract in lowering blood triglycerides in animal models (23,27).
Conclusion
E. billardierei extract can reduce lipid accumulation, triacylglycerol content, and GPDH activity in mature adipocytes. Our findings indicate the suppressive role of the E. billardierei extract on the differentiation of fat precursor mouse cells. Along with other findings indicating the anti-diabetic effects of this extract, the possible role of E. billardierei extract in reducing the incidence of obesity and other metabolic syndrome-like diseases merits more investigation. Further molecular studies are needed to survey the more precise mechanism of this extract.
Acknowledgements
Present study was supported by Baqiyatallah University of Medical Sciences (IR.BMSU.REC.1399.234).
Conflicts of Interest
Authors declared no conflict of interest.
Funding
None Declared.
Received: 2022/12/22 | Accepted: 2023/03/29 | Published: 2023/09/27
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |