BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://journal.zums.ac.ir/article-1-6016-en.html
2- Dept. of Anatomical Sciences, School of Medicine, Kermanshah University of Medical Sciences, Kermanshah, Iran ,
✅ The NAR could recover the liver damage resulting from Isc/R. This impact could be attributed to the antioxidant effects.
Induction of Isc/R in kidney and renal injury can potentially affect the liver (1). Reduced blood supply to an organ or the presence of complete ischemia can easily lead to oxidative stress in tissue similar to the Isc/R process (2). Oxidation occurs through two main pathways, including respiratory burst in WBCs and insufficient oxygen supply to the tissue (3, 4).
Today, it has been proven that many herbal remedies exert therapeutic effects against pathologic conditions through the antioxidant potential (5). Therefore, the flavonoids in plants are known as the main source of antioxidants in Chinese herbal medicine (6). Monoflavonoids and polyflavonoids are two major types of flavonoids (7). The main subsets of natural bioflavonoids are 4ˊ,8”-linkage bioflavonoids and 6ˊ-O,3”,-linkage bioflavonoids (8). Naringenin (NAR) is a natural monoflavone found in grapefruits and tomatoes with proven medicinal properties (9).
Andrade et al. compared the biomedical contents of various species of propolis and found that phenolic compounds have higher antioxidant capacity than flavonoids (10). Moreover, the biochemical constituents and antioxidant properties of purple and yellow Chrysanthemum were analyzed in another study (11) concluding that the yellow type of Chrysanthemum contains high amounts of NAR with fewer antioxidant properties than the purple types (rich in anthocyanin) (12).
Gouveia et al. found higher antioxidant potential for the phenolic part of Chrysanthemum, in comparison with flavonoids (13). Meanwhile, antioxidant features related to flavonoids, such as NAR have been approved. Lou et al. in an experimental study on Kumquat extracts reported high levels of antioxidants associated with phenolic and flavonoid contents. They confirmed some obvious features of NAR in Kumquat extracts, such as removing free radicals and apigenin sorption (14).
Peter et al. compared NAR and the related dimer (i.e., isoginkgetin) in terms of extra Reactive Oxygen Species (ROS). They revealed that isoginkgetin was more effective than NAR in ROS scavenging. However, NAR is changed into dimers in antioxidant reactions (15). Furthermore, in experimental studies, the therapeutic influences of NAR on liver diseases were approved (16). It has been noted that NAR has antioxidant and anti-inflammatory therapeutic impacts on Liver diseases.
Inhibiting the production of interleukin-1 beta (IL-1β) and tumor necrosis factor-alpha (TNF-α) along with enhancing the antioxidant capacity of RAW 134.9 macrophages are known as the major impacts of NAR in the field of antioxidant and anti-inflammation activities (17-19). There are few analytic studies concerning the potential activity of NAR in liver failure following renal Isc/R and no precise outcomes have been noted about the effects of this substance on the expression of apoptotic genes, inflammatory cytokines, and NO levels. Consequently, we investigated the therapeutic effects of NAR on liver physiopathology and inflammation following the induction of renal Isc/R in Wistar rats.
Animals
Sixty-four male Wistar rats aged 12-14 weeks and weighing 250±30 g were prepared from Pasteur Institute, Tehran, Iran. Conditions for keeping laboratory animals based on the ethics of animal care were the temperature of 22±2ºC and photo-cycle of 12 light/12 dark. All treatments on laboratory animals were conducted according to the Ethics Committee of Kermanshah University of Medical Sciences (No.1396.348) (20).
Experiment Design and Surgery
A total of 64 animals were divided into eight groups as follow: 1) control group (0.1% DMSO, surgical procedures with no artery occlusion), 2) Isc/R group (DMSO injection followed by bilateral renal 30-min ischemia and 24-h reperfusion), 3-5) NAR groups (abdominal walls were sutured one hour following laparotomy, gavage with 0.1% DMSO+25, 50, and 100 mg/kg of NAR, 6-8) NAR+Isc/R groups (bilateral renal 30-min ischemia followed by 24-h reperfusion, gavage with DMSO+25, 50, and 100 mg/kg of NAR a day after surgery).
Anesthesia was induced by the intraperitoneal administration of xylazine/ketamine (10/70 mg/kg). The animals were treated for 28 days and were killed by cervical dislocation. Blood samples were attained through needle insertion into the left ventricle. Samples were incubated for 20 min at room temperature and were centrifuged at 3000 rpm for 10 min. The sera were kept at -20°C for enzyme measurements (9). In addition, the liver tissues were dissected after laparotomy for future analysis.
Histomorphometric Assessments
The right lobe of the liver was fixed in 10% buffered formaldehyde. Following the application of the routine histological process, the paraffin blocks were cut as sections of 5 μm (Leica RM2125, Germany) and were stained by H&E. The captures were completed using a microscope (Olympus CZ‑41F 56S02) and Olysia Bio-software (Olympus Optical, Japan) was used for image morphometrical analysis. Subsequently, quantitative histological indices were measured in 50 hepatocytes of each subject, including the diameter of the central hepatic vein, the diameter of hepatocytes, as well as the longest and shortest axis of the cells. A similar technique was performed for the central vein measurements (3).
Liver TAC Level
TAC levels were analyzed by a commercial kit (Cat No: TAC-96A, ZellBioGmbH, Germany) utilizing the colorimetry method. Ascorbic acid was considered a standard. In this kit, sensitivity, diagnostic range, and final absorbance were 0.1 mM, mM 0.125-2, and 540 nm, respectively (21).
NO Level
In this study, the Griess reagent was applied in an acidic medium. First, 10 mg of zinc sulfate was added to 200 μL of the sample. Next, a mixture of 100 μL of sulfonamide, 25 μL of NEDD, and 200 μL of chloride vanadium was added to the previous combination. Following incubation in a dark environment (25oC), the optical density was measured at the wavelength of 540 nm (21).
Inflammatory Cytokines
Radio-immunoprecipitation Assay Lysis Buffer was used for tissue (100 mg) lysis (Abcam, Cambridge, UK) and centrifugation was performed at 30000 g and 4°C for 15 min. Inflammatory biomarkers in the liver homogenate, namely TNFα, IL1β, and TLR4 were analyzed by the enzyme-linked immunosorbent assay ELISA kits (Abcam, Cambridge, UK and MyBioSource, California, USA) and absorbance measurement (16).
Plasma Level of Liver Enzymes
The measured liver enzymes were alanine transaminase (ALT), Aspartate Transaminase (AST), total bilirubin, and albumin as the indicators of liver activity. The latter factors were measured by spectrophotometry kits (Pars Azmun, Tehran, Iran) according to the instructions of the manufacturer (21).
Real-time Polymerase Chain Reaction
Desired genes entailed p53 (F: 50-AAGCTCATTTCCTGGTATG-30/ R:50- CTGCCACAAGAACTAGAGA-30), Bcl2 (F: 50-TGG GATGCCTTTGTGGAACT-30/R: 50-GAGAC AGCCAGGAGAAATCA-30), and Bax (F: 50-ATGGCGAAATGGAGATGAATA-30/ R:50-GCATGGGCATCCTTTAACTC-30). The expression levels of these genes were analyzed utilizing real-time polymerase chain reaction (PCR).
The RNeasy mini kit (Qiagen Co.,Valencia, CA, USA) and RevertAid™ First Strand cDNA Synthesis Kit (Fermentas, Vilnius, Lithuania) were applied for total RNA extraction and cDNA synthesis of liver tissue. The levels of gene expression were measured by glyceraldehyde-3-phosphate dehydrogenase primer
(F: 50- AAGCTCATTTCCTGGTATG-30/
R: 50- CTGCCACAAGAACTAGAGA-30)
as endogenous control and SYBR Green (22).
Statistical Analysis
The one-way ANOVA and Tukey post-hoc tests were used for data analysis by the SPSS software version 16 (SPSS Inc, Chicago, Ill., USA). The final results are shown as Mean±SEM. P-value < 0.05 was considered statistically significant.
Quantitative Histological Parameters
In hepatocytes and central hepatic veins of the experiment groups, the mean diameter was significantly different between the control and Isc/R groups (P<0.05). On the other hand, none of these values had a significant difference between the NAR and control groups (P>0.05). In the NAR and NAR+Isc/R groups, these two morphological features reduced significantly, compared to the Isc/R group (P<0.05) (Figures 1 and 2).
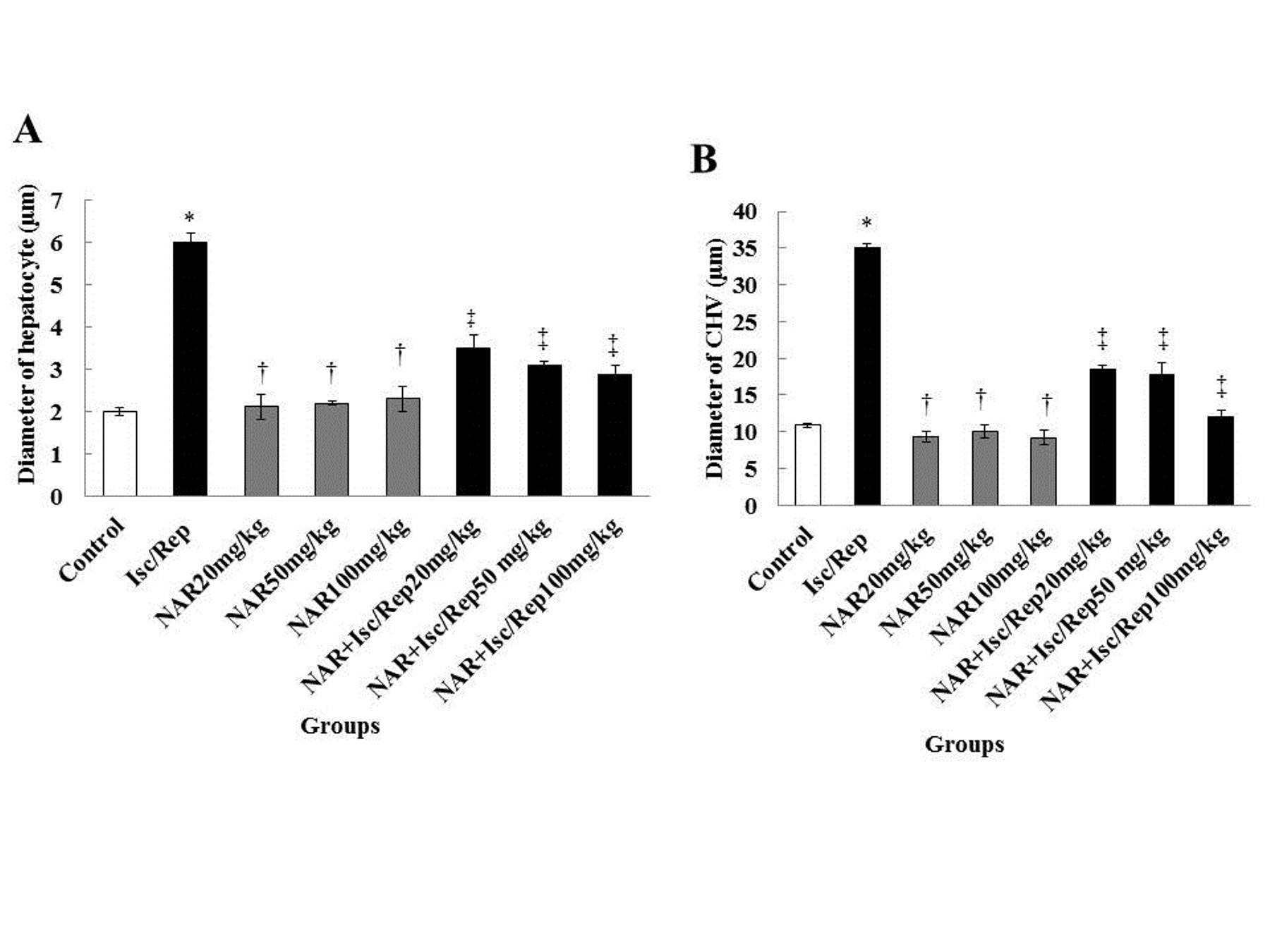
Figure 1. Effect of Isc/R and NAR administration on the diameter of hepatocytes (A), and central hepatic vein (B). *Significant differences between the diameters of hepatocytes and central hepatic vein with the control group (P<0.05). †Significant differences, compared to the Isc/R group (P<0.05). ‡Significant differences, compared to the Isc/R group (P<0.05). CHV: central hepatic vein, NAR: naringenin, Isc/Rep: ischemia-reperfusion
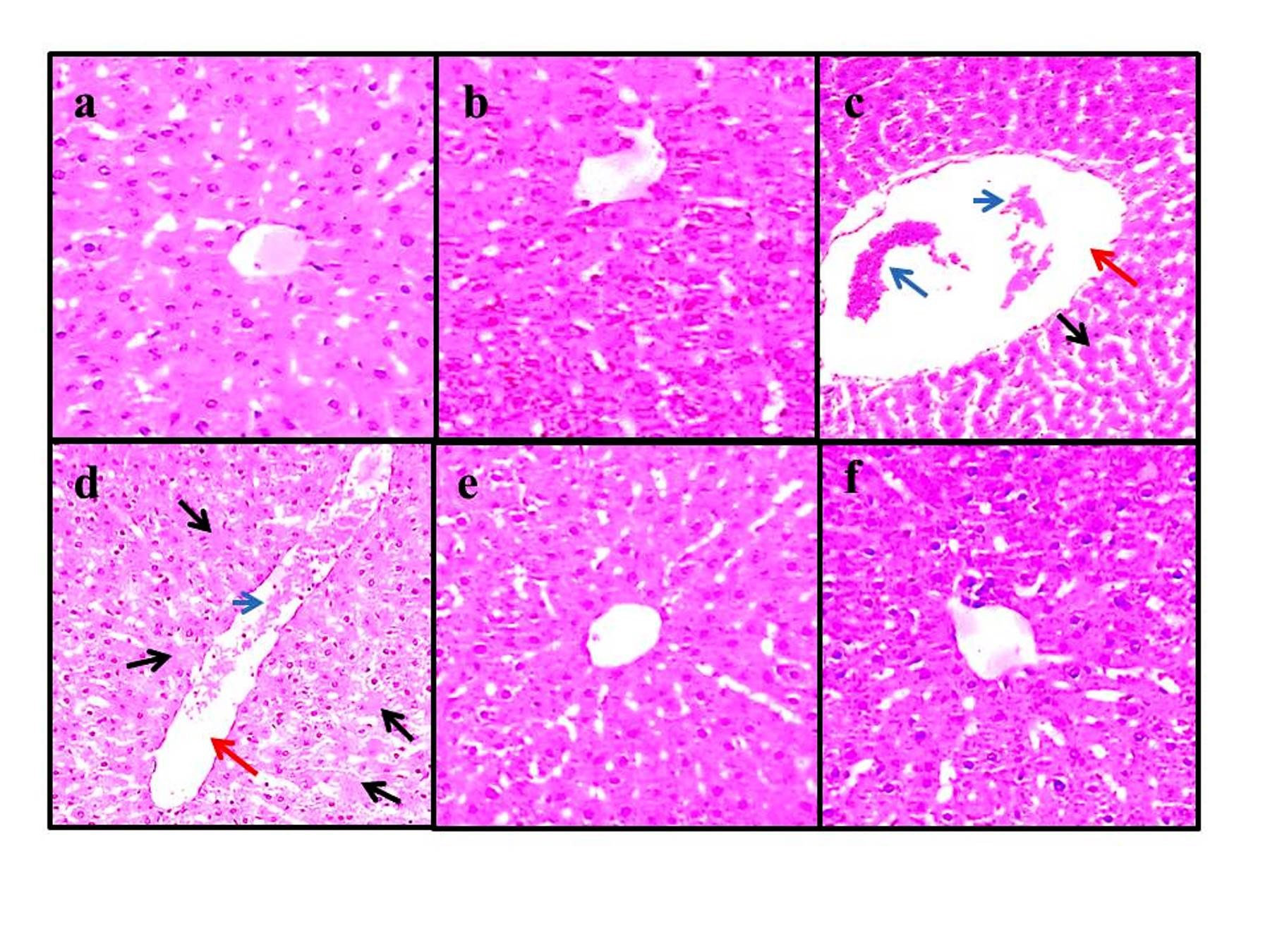
Figure 2. Histological images of the experiment groups (4 µm, H&E, ×100). Groups of control (a), NAR (100 mg/kg) (b), NAR (50 mg/kg)+Isc/R group (e), and NAR (100 mg/kg)+Isc/R group (f). Cell destruction with no nuclei (black arrows), dilatation in the central hepatic vein (red arrows), and hyperemia (blue arrow) in the Isc/R group (c, d). NAR: naringenin, Isc/Rep: ischemia-reperfusion
STAC Levels
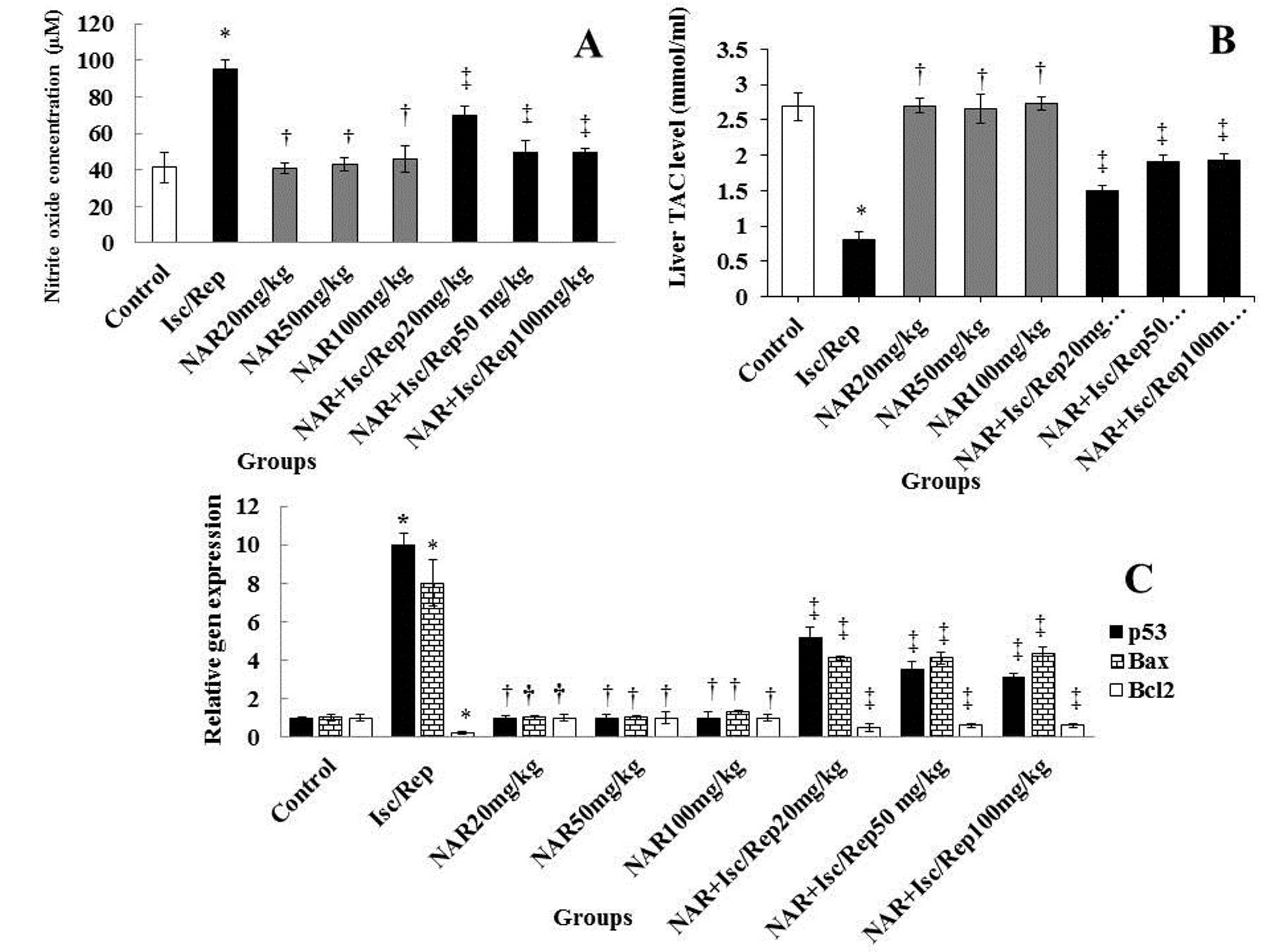
The TAC value of the Isc/R group was observed to be significantly lower than the control group (P<0.05). Furthermore, the TAC level was found to be higher in the NAR and NAR+Isc/R groups than in the Isc/R group (P<0.05). In contrast, no significant effects were found on the TAC level in neither of the NAR groups, compared to the control group (P>0.05) (Figure 3).
NO levels
The level of TAC increased significantly (P<0.05) in the Isc/R group with no significant changes noted in neither of the NAR groups (P>0.05). Moreover, NO levels were shown to be significantly lower in all NAR and NAR+Isc/R groups, compared to the Isc/R group (P<0.05) (Figure 3).
Gene Expression Levels
Up-regulated modifications of apoptotic p53 and Bax genes and down-regulated variations of the Bcl2 gene were identified as significant (P<0.05) in the Isc/R group. Significant down-regulation of p53 and Bax genes and up-regulation of the Bcl2 apoptotic gene were revealed in the NAR and NAR+Isc/R groups, while the alterations were not significant in the Isc/R group (Figure 3).
Inflammatory Cytokines
Levels of inflammatory cytokines were significantly higher in the Isc/R group, in comparison with the control group (P<0.05). The NAR was able to augment the levels of inflammatory cytokines significantly (P<0.05) in the NAR and NAR+Isc/R groups, compared to the Isc/R group. The changes in the levels of inflammatory cytokines were not significant in the NAR group (P>0.05) (Table 1).
Figure 3. Isc/R and NAR affect the levels of NO (A) and TAC (B), and the expression of genes (C). *Significant differences, compared to control (P<0.05). †Significant differences, in comparison with Isc/R (P<0.05). ‡Significant differences with Isc/R (P<0.05). NAR: Naringenin, Isc/Rep: Ischemia-reperfusion, NO: nitric oxide
Table 1. Differences of inflammatory bio-markers among treatment groups
| Enzymes(ng/ml) | Control | Isc/R | NAR | NAR + Isc/R | ||||
| 10mg/kg | 25mg/kg | 50mg/kg | 10mg/kg | 25mg/kg | 50mg/kg | |||
| TNFα | 11.45±1.71 | 51.25±5.03* | 10.07±1.36† | 9.22±1.72† | 10.01±2.64† | 23.4±2.77‡ | 22.1±2.54‡ | 20.3±1.23‡ |
| IL1β | 6.54±0.20 | 25.53±1.31* | 8.08±0.54† | 7.22±0.99† | 7.11±1.01† | 14.7±1.42‡ | 13.3±1.91‡ | 11.7±1.97‡ |
| TLR4 | 0.27±0.01 | 0.81±0.08* | 0.26±0.03† | 0.27±0.04† | 0.26±0.01† | 0.45±0.05‡ | 0.41±0.01‡ | 0.40±0.03‡ |
Mean ± standard deviation was used for data presentation. TNFα: Tumor necrosis factor-alpha, IL1β: Interleukin 1 beta, TLR4: Toll-like receptor 4, NAR: Narigenin, Isc/R: Ischemia-reperfusion. * P-value<0.05 than control. † P-value<0.05 compared to Isc/R group. ‡ P-value<0.05 compared to the Isc/R group.
Liver Functional Enzymes
In the Isc/R group, significant elevation was reported in ALT, AST, and ALP levels (P<0.05). Furthermore, the mean concentration of these enzymes did not change significantly in either NAR groups (P>0.05). The mentioned enzymes were significantly lower in the NAR and NAR+Isc/R groups of all doses than in the Isc/R group (P<0.05) (Table 2).
Table 2. Differences of liver enzymes among treatment groups
| Enzymes(ng/ml) | Control | Isc/R | NAR | NAR + Isc/R | ||||
| 10mg/kg | 25mg/kg | 50mg/kg | 10mg/kg | 25mg/kg | 50mg/kg | |||
| AST | 43.14±2.06 | 132.22±5.78* | 42.09±2.31† | 41.1±2.01† | 41.5±3.23† | 62.7±2.45‡ | 60.2±5.71‡ | 60.3±2.11‡ |
| ALT | 32.43±1.32 | 82.73±5.78* | 32.91±3.36† | 31.7±1.41† | 31±2.07† | 58.4±3.41‡ | 58.8±3.13‡ | 55.1±2.13‡ |
| ALP | 2.81±0.01 | 7.98±0.10* | 2.71±0.01† | 2.22±0.08† | 2.31±2.11† | 4.74±0.72‡ | 4.44±0.34‡ | 3.36±4.11‡ |
Data presentation were as mean ± standard deviation. ALP: Alkaline phosphatase, ALT: Alanine aminotransferase, AST: Aspartate aminotransferase, NAR: Narigenin, Isc/R: Ischemia-reperfusion. * P-value<0.05 compared to the control group. † P-value<0.05 compared to Isc/R group. ‡ P-value<0.05 compared to the Isc/R group.
Discussion
According to the results of this investigation, 60-min renal Isc followed by 5-day Rep resulted in histological liver damages, p53 and Bax genes up-regulation, the disruption of balance in the hepatic enzyme, the induction of imbalance in the liver oxidative-antioxidant system, and significantly elevated inflammatory cytokines. Moreover, inflammation following Isc/Rep induction can lead to hepatic NO generation. The NAR can recover the mentioned damages in the liver by the antioxidant impact.
The findings of the present study showed significant alterations in the histopathology of liver, liver enzymes, and antioxidant balance. In addition, we concluded that NAR is effective in the restoration of these destructive changes. Wang et al. found an increase in the level of serum hepatic enzymes following 1-h Isc/4-h R (23). Furthermore, it was approved by Seifi et al. in a 45-minute treatment (24).
According to the literature, the liver responds to Isc/R by changing the enzymatic content. Therefore, it could be concluded that alterations in the serum level of hepatic enzymes are considered as the main reaction to the renal Isc/R procedure. Reduced Isc and elevated Rep time might diminish hepatic injury (4, 5).
In pulmonary tuberculosis, increased indirect level of alkaline phosphatase (ALP) was detected, while a cholestatic pattern could be diagnosed based on levels of ALP, AST and ALT. Therefore, it can be stated that Isc/R is influenced by the cholestasis pattern of liver damage and liver function. The ability of the normal liver to produce albumin could be considered in this regard (25). The albumin available in blood serum may decline as a result of pathologic conditions affecting the liver following Isc/R. Consequently, albumin could be regarded as a crucial biochemical marker.
Damaged tissue in Isc/R generates excessive amounts of ROS leading to oxidative stress. These excessive ROS are directed to the liver tissue by the bloodstream and cause damage (3). In animals under Isc/R, increased diameters of hepatocytes and central veins were detected, which was approved by other investigations following nicotine administration (26).
Furthermore, in the current study, we demonstrated that NO could lead to the dilatation of the central hepatic vein. The NO plays role in the exacerbation of liver histopathology, such as the increased diameter of the central vein (21). Along with the elevated level of NO in liver tissue, other destructive agents are produced following Isc/R, which can also lead to the vasodilation of the central hepatic vein (27). While NAR can change the pathologic features of the liver to the normal form (28). This confirms the association of histological features with liver function following Isc/R.
The histopathological changes associated with the augmented level of NO could trigger oxidative stress process and acute changes, including steatosis and the atrophy of hepatocytes. It is approved that the previously mentioned histological alterations would disappear gradually with no changes in the volume of hepatocytes and the diameter of central veins (29). Consequently, long-time exposure to NO is critical for toxin elimination leading to the growth of hepatocytes.
In the current investigation, the data revealed significantly increased TLR4 expression in Isc/R rats. Furthermore, NO level and inflammatory biomarkers are correlated in lipopolysaccharide-induced acute lung injuries (30). In the Sertoli cells of rats, activated TLR-5 up-regulates the expression of inflammatory cytokines resulting in diminished testosterone and spermatogenesis (16).
The NAR administration could decrease these pro-inflammatory cytokines with lower influence on TNF-α and higher impact on IL-10 and IL-6 (31). Generally, TLRs, inflammatory cytokines, and NO levels are positively correlated (27). We found significant positive correlations between TNFα, IL 1β, and NO. This result is consistent with the findings of Pari et al., in which a relationship was reported between TLR-4 and IL-5 in cardiomyocyte Hypoxia/Reoxygenation damage (19).
The stimulation of inflammatory cytokines secretion and the presence of TLR could be considered as a general linking cascade, the up-regulation of which is not related to the Isc/R process. In the present study, NAR decreased the hepatic damages caused by Isc/R. The NAR may create protective effects against the pathologic conditions of antioxidants accumulation in the liver. Roshankhah et al. reported that the Petroselinum crispum controlled the damage caused by Isc/R (20). We observed that NAR down-regulated the expression of Bax and p53 apoptotic genes, while up-regulated the Bcl2 gene. The p53 moderates the activity of apoptotic elements, such as Caspase and Bax (32).
It is suggested that Isc/Rep had up-regulatory effects on the apoptotic genes (33). Furthermore, NAR is translocated within the intra-nuclear space to induce the down-regulation of the related genes (34). Bayir et al. reported that the apoptotic genes are expressed significantly in the pheochromocytoma of the adrenal medulla in rats after Isc/Rep (29). In the current study, NAR reduced the expression of Bax and p53 and improved the expression of the Bcl2 gene to prevent cell death.
Conclusion
Acknowledgements
We are grateful to the Research Council of Kermanshah University of Medical Sciences for their financial support.
Ethical considerations
Ethical issues (Including plagiarism, informed consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc.) have been completely observed by the authors.
Funding and support
This research resulted from an independent research without receiving any financial support.
Conflicts of Interest
Authors declared no conflict of interests.
Received: 2020/05/13 | Accepted: 2020/09/5 | Published: 2020/11/14
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |