BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://journal.zums.ac.ir/article-1-6500-en.html


 , Swetha Pasupuleti2
, Swetha Pasupuleti2 

 , Ravikanth Manyam2
, Ravikanth Manyam2 

 , Kishore Moturi3
, Kishore Moturi3 

 , Divya Naga Lakshmi Puvvada3
, Divya Naga Lakshmi Puvvada3 


2- Dept. of Oral Pathology, Vishnu Dental College, Bhimavaram, India
3- Dept. of Oral and Maxillofacial Surgery, Vishnu Dental College, Bhimavaram, India
✅ Arteriovenous malformations (AVMs) of the head and neck are rare vascular benign anomalies but may become lethal when persistent and progressive. It occurs when a fetal capillary bed fails to fully involute, allowing direct connection between arteries and veins. They can also happen as a result of trauma or a hormonal changes.
Vascular malformations are group of birth defects caused by abnormalities in the angiovascular or lymphovascular systems. Arteriovenous malformations (AVMs)are high flow lesions in which there is direct communication between arteries and veins bypassing the capillary bed [1], which are present at birth but can also occur due to any trauma or infection. They can be seen in any region of the body. In the oral cavity, they are mainly seen on the anterior two-thirds of the tongue, lip, palate, gingiva, and buccal mucosa [2]. These can be diagnosed by conventional radiographs, computerized tomograph(CT) scans, magnetic resonance imaging(MRI) or angiographs. Sclerosing drugs and embolization, as well as surgical resection, are used to treat the condition [3,4,5,6].
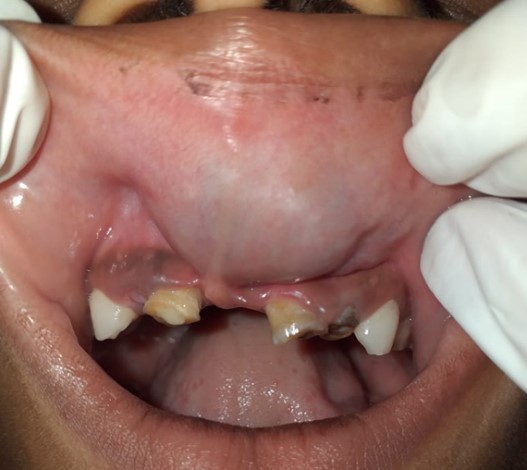
A 7year old female presented to the oral medicine and radiology department, Vishnu dental college, Bhimavaram, with a major complaint of swelling on the upper lip, front tooth region since five years, which began at the age of 8 months due to trauma. The swelling was found to increase in size during crying and coughing. At 16 months of age, she was taken to a medical hospital, Bhimavaram, where she was assured that swelling would decrease in size gradually (Figure 1).
The Ultrasonography revealed a defined triangular-shaped hypoechoic lesion with irregular margins measuring 1× 0.5cm noted in the upper lip predominantly on the left side from the midline. As to rule out vascular flow of the lesion MRI and CT angiogram was advised.
MRI revealed a T1 weighted image. The sagittal section with a hyperintense area seen on the upper lip with no local flow of voids and axial section reveals a 2× 1.5 cm hyperintense area with no bone involvement. (Figure 2).
CT angiography of the neck showed an ill-defined soft tissue mass measuring approximately 2.8× 1.2cm noted in the midline upper lip region with three internal calcific foci. (Figure 3).
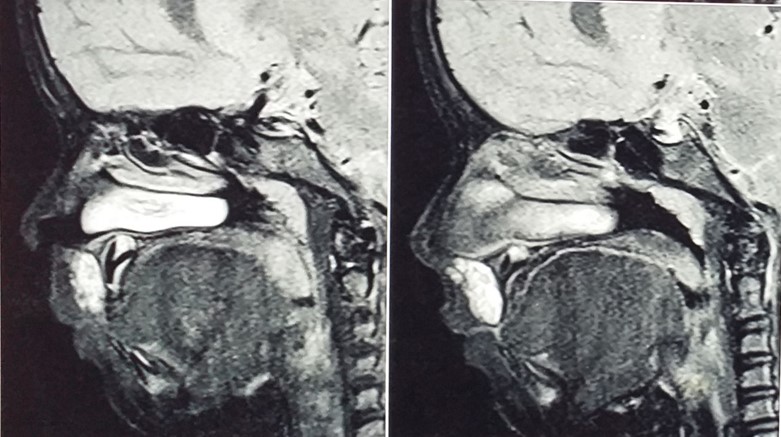
Figure 2. MRI (Sagittal view) T1 weighed image showing hyper intense area on upper lip.
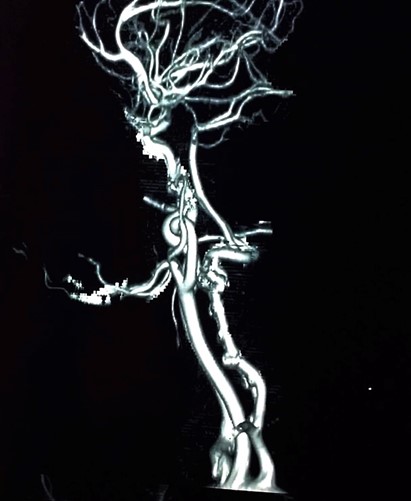
Figure 3. CT angiography of the neck showed an ill-defined soft tissue mass in the midline upper lip region with three internal calcifications.
The final impression was given as a slow flow vascular malformation and differential diagnosis of slow flow hemangioma and arteriovenous malformation. Intralesional corticosteroid Kenalog 40 mg was administered under local anaesthesia, and follow up of the patient was recommended. The lesion was excised under general anaesthesia. The feeder vessel was blocked with the sutures, and then the mucosal incision was given from 51 to 62, and the entire lesion was dissected. A bright red mass of 4 ×3 cm of size, irregular in shape where the surface was hard and rubbery in consistency.
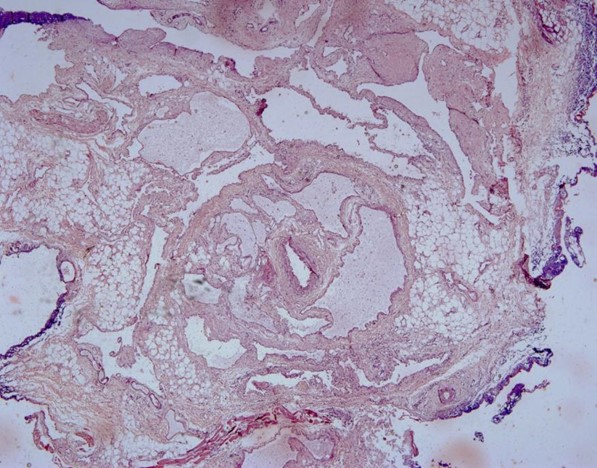
Histologically, in 4x dilated venules with thin endothelial lining engorged with RBC's and dilated arterioles are seen. Under 20x dense fibrous connective tissue consists of dilated arterioles and venules with flat endothelial lining. Numerous small blood capillaries were seen, indicating a highly vascular lesion. Under 40x dilated venules arterioles which are lined by thin to flat endothelial cells. After considering all features, the lesion was identified as AVM of the upper lip. After one month, the patient was examined and the surgical incision had healed entirely.(Figures 4,5).
 |
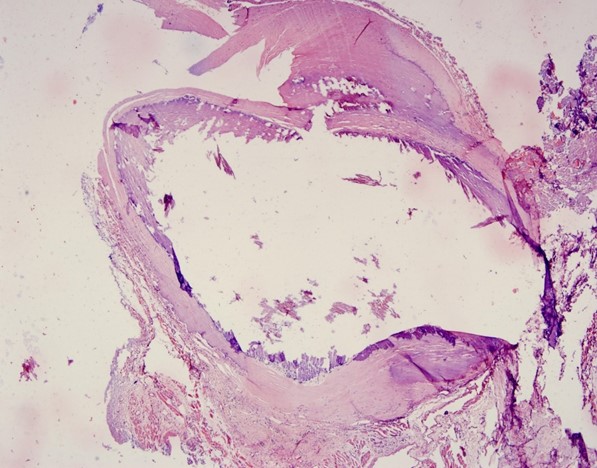 |
| Figure 4. 4x view showing dilated arterioles and venules with engorged RBC’S | Figure 5. 20x view showing Phlebolith |
Discussion
Vascular malformations are due to errors in morphogenesis present at birth, increasing in size with the child's age and are evident after any infections, trauma, or any surgeries and do not involute. They may be a low flow i.e., (Capillary, Lymphatic, Venous) and high flow lesions (arterial) or Arteriovenous (AVM), lymphatico venous (LVM), capillary lymphaticovenous malformations (CLVM) [7].
AVMs are high-flow intravascular slow-growing lesions caused by a failure to complete a capillary bed, leading to a direct connection between arteries and veins, which are reported to occur in 0.1% involving the head and neck region. Congenital AVMs are present at birth but evident during the 2nd or 3rd decades of life. Acquired AVMs are often post-traumatic with a prior history of trauma, surgery, and hormonal changes at the time of puberty or pregnancy [8].
Etiopathogenesis
Congenital or Familial AVMs:
Most AVMs are sporadic, however a few hereditary disorders, such as Cobb syndrome, Parkes Weber syndrome, Bonnet-Dechaume-Blanc syndrome, or Wyburn-Mason syndrome, are encountered in conjunction with AVMs [9]. Two main prevailing theories for the pathogenesis of AVMs, i.e., TGF-beta signalling deficiencies and the genetic two-hit hypothesis A mutation in the RASA1 gene, which expresses p120- Ras GAP on chromosome 5q, has been found in families with congenital deformities. Defects in endothelial cell ligands or receptors can result in the development of AVMs. In proliferating AVMs, estradiol-17 beta-receptors are found[5].
Acquired AVM's:
Acquired AVM's occur due to trauma, after surgery, ischemia due to thrombosis, or due to hormonal changes (puberty and pregnancy). When compared to congenital aetiology, the lesion is frequently fed by a single vessel as a result of trauma [8]. In our situation, the lesion is the result of trauma.
Clinical features
The incidence of AVM's is approximately 40-60% of lesions which are apparent at birth and about 30% of AVM's are clinically apparent during childhood, occurs equal frequency in both the sexes [10]. Most frequent in the second and third decades of life. Kohout et al. reported on 81AVMs situated in the head and neck area, the most of which were positioned over the cheek (31%), ear (16%), nose (10%), forehead (10%), upper lip (7%), mandible (5%), neck (5%), scalp (4%), and maxilla (4%) [11]. In the oral cavity, these are mainly seen in anterior 2/3rd s of tongue, lips, palate, gingiva and buccal mucosa [2]. Intraosseous involvement is seen in 35% of cases. A flat or raised reddish-blue localized compressible soft tissue swelling with a bleeding tendency is seen. Vascular nevi or phleboliths may also be seen discolouring the adjacent skin or mucosa. In our case, the swelling was bluish-purple due to phlebolith's presence [11]. A palpable thrill or bruit may be felt. Occasional local hyperthermia may be seen. Blood shunting lowers nutritive flow, which can lead to skin necrosis, ulceration, and bleeding. AVMs can proceed through four stages and can be graded based on severity utilising the ISSVA-accepted Schobinger 1990 staging method. StageI(quiescence), StageII(Expansion), StageIII(Destruction) and Stage IV (Decompensation) [10].
Imaging features
Diagnosis is usually made from clinical examination alone. AVM's differentiating features will be palpable thrill, warmth, pulse due to its high vascular flow. Imaging findings will rule out the AVMs from vascular malformations. In OPG, calcifications are rare. Phleboliths, appear as round multiple radiopaque masses in concentric rings with smooth periphery giving it laminated or bulls-eye appearance [12]. Ultrasonography differentiates between a low flow and high flow lesion [13]. CT scan can delineate the extent of involvement. It may not always differentiate and trace the AVMs from VMs. MRI may help depict the extent of the lesion's invasion, distinguish between high flow and low flow lesions by providing multiplanar images. Angiography is required for poorly defined cases and embolization prior to surgery, since it reveals major feeding vessels flow characteristics of AVM & anastomoses [14].
Histopathological features
The majority of AVMs are non-encapsulating aggregations of blood vessels in the submucosal tissues. The vascular complex has abnormally thick and thin-walled arteries and veins. The confluence of these blood arteries in connective tissue suggests shunting between arteries and veins, lined by a single flat endothelial lining. The internal elastic lamina of arteries can be duplicated, interrupted, and deformed. Feeder arteries and veins may be visible in close proximity. Stromal calcifications or focal thrombosis, atherosclerosis may also be seen [15].
Differential diagnosis
Hemangioma and other VMs, as well as other neoplasms such as hematoma and pyogenic granuloma, are included in the differential diagnosis of AVMs. In a hemangioma, endothelial cells expand rapidly at first, followed by spontaneous involution, endothelial cell proliferation, and a rise in the number of mast cells infiltrate. In contrast, AVMs are persistent lesions with no spontaneous involution, no endothelial cell proliferation, and the normal mast cell count is seen [16]. Hematoma and pyogenic granuloma may be included in the differential diagnosis of non-pulsatile AVM, and histopathologically, they do not reveal a combination of arterioles and venules.
Treatment
Because of their hemodynamic characteristics, AVMs present a therapeutic challenge. They don't require treatment if asymptomatic. Multimodal approach of laser therapy followed by embolization and surgical resection [17]. Embolization is accomplished by injecting glue or another non-reactive liquid adhesive substance into the AVM to block it off. Sodium morrhuate, hot water, nitrogen mustard, 1 percent sodium tetradecyl sulphate, and other sclerosing agents have been used to treat high flow lesions but have proven ineffectual because they were driven from the site of infection by the speed of blood flow. Because of their coagulative properties, high power lasers are an attractive therapeutic choice. Embolization using polyvinyl alcohol particles, gel foam, coils, methyl methacrylate, and silicone spheres lowers hypervascularity and hence facilitates surgical resection. Embolization by polyvinyl alcohol particles, gel foam, coils, methyl methacrylate, and silicone spheres reduces the hypervascularity, therefore aids in surgical resection [18]. The modalities described above have significant drawbacks.Excessive fibrosis, scarring, bleeding, and aesthetic concerns are all factors to consider [19,20]. Surgical resection remains the gold standard treatment for AVMs in children [21], in this case we have performed surgical resection to prevent any adverse effects.
Conclusion
corticosteroid injection (Kenalog 40mg) was initially administered as an alternative approach for managing vascular lesions which was followed by surgical excision. It depends on the patient's age, as well as the location and size of the lesion, whether they should be followed up on or treated. Because of the longterm impact, and functional inadequacy with steroids and radiation, surgical excision and cryosurgery for tiny lesions and superficial wounds, is successful.
Acknowledgements
None.
Conflicts of Interest
The authors declare that they have no conflict of interests.
Received: 2021/04/12 | Accepted: 2021/10/17 | Published: 2022/08/8
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |