BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://journal.zums.ac.ir/article-1-6061-en.html


 , Omid Akhiani2
, Omid Akhiani2 

 , Hooman Ravaei3
, Hooman Ravaei3 

 , Seyed-Ehsan Beladian-Behbahan4
, Seyed-Ehsan Beladian-Behbahan4 

 , Gholamali Javdan *5
, Gholamali Javdan *5 


2- Dept. of Pharmacodynamics and Biopharmacy, Faculty of Pharmacy, University of Szeged, Szeged, Hungary
3- Young Researchers and Elite Club, Ardabil Branch, Islamic Azad University, Ardabil, Iran
4- Dept. of Health and Social Medicine, School of Medicine, Shahid Beheshti University of Medical Science, Tehran, Iran
5- Minimally Invasive Surgery Research Center, Iran University of Medical Sciences, Tehran, Iran ,
✅The results of this study showed that treatment with nano-selenium oxide reduced flap tissue necrosis and lipid peroxidation significantly; it also increased SOD activity. Therefore, the survival of the flap and its efficacy increased.
Skin flaps are one of the most important reconstructive treatments for skin injuries caused by surgery, trauma, or tumor removal. After extensive skin damage, which the body is unable to repair, flaps are used to maintain skin function and repair the defects; they maintain the skin integrity (1). The advantage of skin flaps is the greater coordination of such flaps in terms of color, consistency, and thickness with the original tissue compared to other restorative methods. Their importance is due to the simplicity of the technique and the homogeneity of the flap tissue with the patient’s body. The flap is much easier tolerated by the body and is safe from rejection reactions. The speed of recovery in the skin flap method is high, which directly affects the patient’s quality of life (2).
Skin flaps are divided into different categories. If the tissue of the flap stays attached to its source from one side, it is called a pedicled flap (3). The connected area is called the pedicle, which is a tissue perfusion site and flap connection to the body. Pedicled flaps can be used to repair the area around the source of these flaps (4).
Despite the applicability of the skin flap method, the distal region of them is impaired due to tissue malperfusion. About 1%-5% of the flaps face entire flap necrosis and tissue loss; one-third of them also develop partial necrosis. Therefore, controlling flap tissue loss and increasing flap viability are important as much as this treatment method; they make the prognosis of the flap method much better (5, 6).
Survival of skin flaps, especially their distal area, is associated with serious risks, such as insufficient tissue perfusion, insufficient oxygen supply, low excretion and residual tissue waste, tissue loss by necrosis and apoptosis, ischemic injury and damage caused by reactive oxygen species (ROS) and oxidative stress (OS), and inflammatory processes of the immune system (4). According to some studies, metabolic changes, such as decreased oxygen, glucose, adenosine triphosphate (ATP), superoxide dismutase (SOD) and increased CO2, lactic acid, prostacyclin, thromboxane, and superoxide radicals, are signs of cited problems (1, 7, 8).
Among the factors that threaten flap survival, the phenomenon of ischemia-reperfusion injury (IRI) is one of the main mechanisms of damage. OS and inflammatory processes of the immune system, followed by IRI, also cause damages (3). OS causes direct cell destruction (necrosis) or activation of the planned cell death process (apoptosis) by damaging the mitochondrial membrane.
After skin flap formation, the tissue undergoes a type of ischemia; it is due to the tissue perfusion, which is only established from the base of the flap. With time passing and new vessel formation, tissue perfusion is re-established, which is called reperfusion (3, 4, 6). Initially, this phenomenon was considered to be consistent with tissue life, but studies have shown that by re-establishing blood flow, molecular pathways produce ROS, which can cause lipid peroxidation, endothelial, membrane, and DNA damage, and activation of necrosis and apoptosis mechanisms (3, 4, 6).
OS is also caused by the inflammatory activity of the immune system. Some factors that influence cell surface necrosis are as follows: radical reaction of superoxides with the endothelial layer, membrane processes, and oxidation of membrane fats; disruption of the membrane transfer process and cell permeability; and neutrophil migration (3, 4, 6).
Accordingly, extensive studies have been conducted on OS neutralization and reduction in flap tissues. Extensive research has been done on tissues such as the kidney (9, 10), brain (11, 12), and skin. In the mentioned organs, enhancement of system antioxidant power is as important as apoptosis mediators and ROS load reduction (13, 14). Various substances and drugs have been used in different studies to maintain the survival of skin flaps, the most important of which are antioxidants and plant extracts, which significantly affect flap survival maintenance, OS, and necrosis reduction (3, 6, 15-17).
Selenium is also known as an antioxidant that protects cells from damage by free radicals, such as oxygen radicals, which oxidizes adjacent molecules. Selenium is an essential element in the body (non-metallic and rare with atomic number 34). It is available in the form of selenoprotein in combination with amino acids in the form of selenocysteine and selenomethionine (18).
The relationship between selenium and human health can be explained by its various functions, such as antioxidant, antitumor, and immunity regulation properties. As one of the main functions, it prevents oxidative damage (especially to DNA) by destroying free radicals (18, 19) and as an integral part of the glutathione peroxidase and thioredoxin reductase enzymes; actually, it acts as a powerful antioxidant (20).
Numerous studies on various tissues (3, 21, 22) have shown that natural substances or extracts are widely used for synthetic drug production; whatever they are, if they produce antioxidant effects or strengthen the body’s immune system against OS, it will lead to reducing cell death and tissue loss (4, 6). Given that selenium has shown similar effects, it can be expected to play a role to counteract OS (23, 24). In addition, it plays an important role in different physiological processes, such as improving fertility (20), reducing drug toxicity, regulating thyroid and proper immune system function (18), improving cardiovascular health, and preventing neuronal degeneration and cancer progression. As a result, it is known to be an important factor to fight against diseases. Selenium deficiency also has a negative effect on proper protein folding and calcium loss (25).
Selenium is more commonly used in nanotechnology (26) in combination with other minerals in the synthesis of other applications, such as selenium-zinc, selenium-cadmium, and selenium-lead; it is used in imaging and diagnostic studies (27). Recently, in discussing the properties of selenium, selenium nanoparticles have been noticed as a possible source of selenium in dietary supplements; it has several prominent advantages, such as chemical stability, biocompatibility, and low toxicity (28).
Nanoparticle systems are a promising alternative for oral delivery and dietary supplements. Nano-selenium has significant anti-inflammatory and anti-cancer activity; it also has high potential in chemotherapy and drug delivery. The present study aimed to investigate the selenium oxide effect on improving skin damage caused by skin flap in the rat. We also tried to evaluate the antioxidant effects of nano-selenium on survival maintenance and destruction reduction of skin flaps; they determine the possibility of using this substance to increase the efficiency of skin flaps.
In this experiment, 30 male Sprague-Dawley rats weighing between 200-250 g were randomly divided into three groups (10 rats in each group). During the study, the rats were placed in single cages with free access to water and food. All stages of the study were conducted in accordance with the principles of the study on laboratory animals and the guidelines of the Ministry of Health.
This study was approved by the Ethics Committee of Tehran University of Medical Sciences (ethical code: IR.IAU.PS.REC.1397.395); it was conducted at the Research Center for Dermatology and Liver Diseases. The animals were anesthetized by the injection of pentobarbital sodium 50 mg intraperitoneally. We used McFarlane et al.’s procedure to perform skin flap surgery and measure the necrotic area. The animals were shaved, and the area was treated with antiseptic alcohol. An hour before flap surgery, the nano-selenium was injected intraperitoneally. Then, after 7 days, the animals were scarified with ketamine (150 mg/kg) to continue the experiments. The experimental groups are described in Table 1.
Table 1. The experimental groups
| Order | Groups | Procedure |
| 1 | Sham | In this group, only incisions were made at the edge of the surgical site, but the skin flap did not elevate from its bed. |
| 2 | Flap surgery group | In this group, after making incisions on skin, the skin flap was elevated from its bed and a piece of plastic film was placed under the skin. |
| 3 | Flap Surgery Group + Treatment of nano-selenium oxide | In this group, after incision and lifting the flap, nano-selenium oxide treatment was used with a dose of 25 mg / kg (intraperitoneally). |
Flap Surgery Procedure
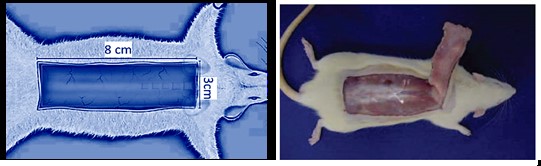
We used McFarlane et al.’s procedure to elevate the skin flap and measure the necrotic area (29). An hour after using the drug, two parallel incisions were made; in the next step, the distal ends of two parallel incisions were connected, and a 3 cm width base was created to supply blood to the body. After lifting the flap and elevating from the lower fascia, an impenetrable plastic barrier similar to the flap size was placed between the flap and its corresponding bed; it prevented the flap from feeding and blending with the bed (see Figure 1).
Figure 1. McFarlane et al.’s method of creating skin flaps on the dorsal surface of the rat.
Method of Calculating Flap Necrosis Percentage
On the seventh day after the operation, the percentage of the necrotic area of the flap was calculated using the paper pattern method. The boundary between living and necrotic tissue was considered to be a sign of necrosis due to skin color, skin softness, heat and having hair (as two markers for skin survival and opacity), and firmness and coolness. To calculate the percentage of the necrotic area, a mold was drawn from the entire flap; a transparent paper in the equal size with mold (representing live skin and skin and necrosis) was cut and weighed. Then, the necrosis rate was determined on a transparent paper pattern; it was cut and weighed too. By dividing the weight of the necrotic flop paper template on the total weight of the flop surface paper template, the percentage of the flop surface necrosis was obtained.
Method of Malondialdehyde Measurement
The level of malondialdehyde (MDA) was used as an indicator of lipid peroxidation, which indicated the amount of damage caused by oxygen free radicals. MDA was measured in tissue removed from the flap. The samples taken from the skin flap were placed in a phosphate buffer and homogenized; finally, a suspension was obtained. It was centrifuged (300 g for 10 minutes), and the prepared solutions were evaluated. Thiobarbituric acid was added to the suspension; the red color (which was caused by the MDA reaction to Thiobarbituric acid) was read at a wavelength of 532 nm (1, 3, 6).
Method of Measuring SOD Activity
First, 920 μL of assay buffer was poured into a cuvette, 40 μL of the sample was prepared, and 40 μL of hematoxylin was added. The above material was mixed very quickly, and the light absorption rate was recorded at 560 nm every 10 seconds for 5 minutes. The inhibition percentage was obtained using the formulas in the kit instruction. The inhibition percentage was applied to the standard curve, and the activity was reported as nmol/mg protein per international unit (3, 6).
Statistical Analysis
The results were analyzed in the three experimental groups using 1-way analysis of variance (ANOVA); the groups were also compared with each other by the Tukey test. Prism statistical software was used to analyze the data. P-value<0.05 was considered as a significant level (1, 3, 6).
The Percentage of Flap Necrosis
The percentage of flap necrosis (see Table 1 and Figure 2) was not considerable in the sham group (about 7.8%), but it was increased in the flap surgery group to 52.1%. This change was significant compared to the Sham group (P<0.05). In the third group (flap surgery+nano-selenium oxide treatment), in which rats were treated with nano-selenium oxide, the necrosis rate of the flap was reduced to 31.06%; it also showed a significant decrease compared to other groups (P<0.05).
Table 2. The values of the measured variables in the experimental groups
| Percentage of tissue necrosis after ischemia-reperfusion of skin in the rats treated with nano-selenium oxide | |
| Groups | Tissue Necrosis (%) |
| Sham | 7.8±1.02 |
| Flap surgery group | 52.1±4.8 * |
| Flap Surgery Group + Treatment of nano selenium oxide | 31.06±4.1 ** |
| Tissue SOD activity (nmol•mg-1.tissue) | |
| Groups | SOD activity |
| Sham | 42.01±7.1 |
| Flap surgery group | 15.09±1.8 *** |
| Flap Surgery Group + Treatment of nano- selenium oxide | 67.03±10.03 *** |
| Tissue malondialdehyde (MDA) (nmol•mg-1.tissue) | |
| Groups | MDA level |
| Sham | 24.2±4.21 |
| Flap surgery group | 80.0±14.4 * |
| Flap Surgery Group + Treatment of nano- selenium oxide | 42.08±5.82 ** |
* Flap necrosis and MDA level in the Flap surgery group, significantly increased in comparison to Sham group (P<0.05).
** Flap necrosis and MDA level in the Nano-selenium oxide-treated group, significantly decreased in comparison to the Flap surgery group (P<0.05).
*** SOD activity significantly decreased in the Flap surgery group in comparison to the Sham group (P<0.05); it increased in the Nano- selenium oxide-treated group compared to the Flap surgery group (P<0.05).

Figure 2. The flap necrosis percentage, SOD activity, and MDA level diagram among the experimental groups.
Abbreviations: SOD, superoxide dismutase; MDA, malondialdehyde.
*** is equal to (P<0.05).

Figure 3. Diagram of the percentage of tissue loss and the percentage of flap survival, SOD activity and MDA level among the experimental groups.
Abbreviation: MDA, malondialdehyde.
In the approximation diagram (Figure 3), the amount of tissue loss in the two groups, which the flap was elevated, was closer to each other; the related triangle to the lost tissue is shifted to the bottom. The point of the flap surgery group is also more centrifugal than those of the other groups (more lost tissue). In the triangle related to the percentage of tissue survival, the survival rate was at the highest level, when the tissue had not been elevated from its bed. In the flap surgery group and surgery group+nano-selenium treatment, the triangle vertices became more centralized due to less survival.
SOD Activity
The activity of the SOD enzyme was measured because it can be as a marker that indicates the antioxidant capacity of the tissue (see Table 1 and Figure 2). It decreased from 42.01 to 15.09 in the flap surgery group compared to the sham group (P<0.05). In the group treated with nano-selenium oxide, the SOD enzyme activity increased to 67.03; it was statistically significant compared to the flap surgery group (P<0.05).
In the SOD enzyme activity approximation diagram (Figure 3), the most centrifugal center of the triangle belongs to the group treated with nano-selenium oxide; it shows the effectiveness of treatment. The triangle shift, which is farther away from the flap surgery group, shows the profound effect of surgery (without treatment with nano-selenium oxide) on reducing the antioxidant capacity of the tissue.
MDA Level
This metabolite is an indicator of lipid peroxidation; its increase in the tissues is a sign of tissue damage and the production of free radicals. According to Table 1 and Figure 2, MDA increased to 80.2 in the flap surgery group; it was statistically significant compared to the sham group (P<0.05). MDA in the sham group was 24.2. It decreased again and reached 42.08 (about half of the MDA level in the flap surgery group) in the group of flap surgery+treatment of nano-selenium oxide.
In the approximation diagram of MDA (Figure 3), the highest rate of lipid peroxidation belonged to the flap surgery group. However, in the other two groups, the vertices of the triangle are more centrist; the proximity to the center indicates a decrease in lipid peroxidation in the flap surgery group+treatment with nano-selenium oxide
Discussion
Skin flaps have a great role in reconstructive surgery to treat skin defects. However, the survival rate of skin flaps, especially in the distal area, is greatly affected by the factors such as ischemia-recurrence, ROS, and OS. These factors cause tissue necrosis and tissue loss and even activate cellular apoptotic mechanisms; they also alter Bax and Bcl-2 pathways. Therefore, effective protective therapies should be used to maintain flap viability to have highly efficient skin flaps.
One of the most important treatments for flap survival is the use of antioxidants and substances that increase the total antioxidant capacity (TAC) of the tissue. There is a lot of physiological complexity to affect oxidation reactions, but in any way that OS can be reduced, necrosis also can be reduced, and viability of the skin flap can be increased.
Existing chemical drugs can help reduce OS, but they are often associated with side effects; meanwhile, nanoparticles are more important because of advantages such as more bioavailability and less toxicity. In this study, we also examined the effect of nano-selenium oxide on the survival rate and OS of skin flap tissue.
Selenium is an element that has received a lot of attention in recent studies due to its significant antioxidant properties. Researches have shown that proteins containing selenium (selenoproteins) play an important role in chronic inflammation and the onset of immune responses; they are known to be good anti-cancer agents. According to the literature, selenium has been widely used as an important dietary supplement in the fields of food, medicine, and pharmaceuticals, especially because of its association with the immune system and cancer processes (28, 30).
Various mineral and organic varieties of selenium, such as sodium selenite, selenium chloride, selenomethionine, and selenocysteine, are also used as a dietary supplement along with various medications in doses of less than 200 μg/d for adults (31). These various mineral forms act as strong antioxidants, anti-cancer agents, and anti-aging agents; they are also known to protect humans against muscular dystrophy (32).
A study found that selenium reduced ROS in the cells and tissues of an animal model, which generally confirmed our conclusions about OS reduction cells (33). Another study in the field of selenium hydroxyapatite nanoparticles reported the anti-cancer effects of selenium (34).
In a study (35) on antioxidant effects of selenium nanoparticles, a number of rats were divided into six groups as follows: 1) control group, 2) selenite group, 3) nano-selenium group, 4) lead-poisoned group, 5) selenite+lead poisoning group, and 6) nano-selenium group+lead poisoning group. OS markers were measured in the rats serum 35 days after the start of the cited study. SOD enzyme activity decreased in the lead-poisoned group. In our study, in the group of flap surgery, the amount of SOD decreased too (P<0.005); our findings are consistent with this study (in the OS condition and SOD decreases).
The activity of the SOD enzyme is an indicator of antioxidant capacity. Therefore, it can be concluded that in IRI conditions of flap tissues, the self-reactive activity of the tissue against ROS is also reduced. In a study by Akil M et al., selenium increased SOD in rats under OS caused by activity; it was consistent with our results (12).
In a study by Varoglu and colleagues in 2009 (36) on the effects of selenium on blood flow in burns caused injury, they declared that selenium significantly increased blood flow to the wound; also, it can provide better tissue perfusion and decrease the rate of necrosis. A study in 2017 by Hasanvand et al. found that in IRI of the renal tissue, selenium significantly reduced MDA levels (10). Regarding our results, selenium reduced the amount of MDA as an indicator of lipid peroxidation in the treated group with nano-selenium oxide.
In a study by Shanu and colleagues in 2013 (37), dietary selenite was used in a mouse model with acute renal impairment due to rhabdomyolysis. They stated that dietary selenium significantly reduced lipid peroxidation in the kidney.
Our finding indicated that nano-selenium oxide decreased the necrosis rate of skin flaps significantly; the survival rate of flap tissue increased consequently.
Conclusion
In general, the results of our research and other researches showed that selenium can have noticeable antioxidant effects on reducing the subsequent free oxidation of IRI. According to our findings, it could be concluded that selenium can significantly reduce OS; it leads to MDA decrease and tissue inherent antioxidant capacity increase. Occurred physiological processes lead to SOD increment; as a result, the necrosis of the flap is reversely correlated with survival.
This study showed that selenium has the potential to be used clinically to increase the survival of skin flaps, reduce flaps’ OS, and increase the inherent antioxidant capacity of flap tissue. However, more researches are needed. It is recommended to measure the markers that are more indicative for OS.
Acknowledgements
None.
Conflicts of Interest
The authors declared no conflict of interest.
Received: 2020/06/13 | Accepted: 2021/02/9 | Published: 2021/02/28
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |