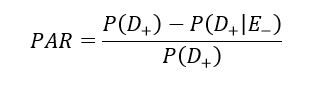BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://journal.zums.ac.ir/article-1-6057-en.html
2- Research Center of Prevention & Epidemiology of Non-Communicable Diseases, Shahid Sadoughi University of Medical Sciences, Yazd, Iran ,
3- Yazd Cardiovascular Research Center, Shahid Sadoughi University of Medical Sciences, Yazd, Iran
4- Dept. of Health Promotion, School of Health, Shiraz University of Medical Sciences, Shiraz, Iran
✅ Age cannot be controlled so that CVDs are mainly attributed to high blood pressure. Therefore, blood pressure control should be considered as a primary strategy to reduce CVD incidence.
Cardiovascular diseases (CVDs) are among the major causes of mortality and disability around the world. They are one of the most important health issues in both developed and developing communities (1). CVDs can reduce life quality and impose great expenditures (2). Based on the Global Burden of Disease study (2017), CVDs account for 31.8% of all mortalities; this measure was reported to be 39.2% for the Middle East and north of Africa nearly. Among these countries, the highest CVDs-associated mortality rate belonged to Tunisia (51.5%), followed by Egypt (46.6%) and Iran (42.5%) (3). In Iran, it is estimated that almost 15 million individuals suffer from CVDs; they occupied the largest number of critical care unit (CCU) beds in 2009. Nowadays, CVDs are the main cause of mortality in Iran too, such a way that nearly 360 out of 800 daily mortalities are related to them (4). Overall, CVDs follow an ascending trend in the world and particularly developing countries. The increase in CVD prevalence may be attributed to its risk factor increment and inappropriate interventions (5). No proper management has been applied yet.
Population attributable risk (PAR) is one of the important public health parameters in the epidemiologic study, which measures the effects of risk factors on general health (6); it expresses the prevention possibility. This parameter determines whether the disease risk is decreased in case of risk factor exposure elimination in the target population (7). In recent years, PAR has been vastly used in general health studies to investigate the relationship between diseases and risk factors. PAR is calculated using the following formula:
D (+/-) represents the disease status, and E (+/-) indicates the exposure status (8). Identifying risk factors that are forcefully associated with increased risk and are highly prevalent would help to understand the potential causes of a large portion of CVDs (9).
This study computed the PARs of CVDs risk factors. In other words, it aimed to determine the association of identified risk factors and CVDs prevalence in the target population (6). The results will help policymakers to make proper decisions to reduce CVDs prevalence; considering the great costs of CVDs, primary prevention is important, concerning cost-effectiveness (10).
In this study, the used data belonged to the recruitment phase of the Shahedieh Cohort Study, conducted in Shahedieh city, Yazd Province, Iran. Shahedieh study is a part of the Persian Cohort Study and has been started since 2016; it has been designed to investigate chronic diseases among 10 000 adults in Shahedieh and annexed cities. This region was selected due to the accessibility of individuals, limited migration to other cities, homogeneous ethnicity, and individuals’ cooperation willingness. Data were recorded by trained individuals. At first, the subjects aged above 35 years were invited to the cohort study center or visited at home by researchers. It should be noted that participation in the research was voluntary.
In this survey, the data of 9852 people were used. These data were collected through a questionnaire, clinical examinations, and urine, blood, and paraclinical tests. The studied variables included demographic characteristics, history of chronic diseases, medications, pulse pressure, sleeping status, physical exercise, personal habits, and anthropometric-listed data by a practitioner (11). Moreover, CVDs were diagnosed and investigated precisely by specialists.
Physical exercise was estimated via the International Physical Activity Questionnaire (IPAQ). This questionnaire has been utilized in numerous countries, and its validity and reliability have been confirmed in Iran. In a study performed by Baghiani Moghadam et al., Spearman’s correlation coefficient of the questionnaire was obtained as 0.9, which was comparable to the reliability of the original version. Besides, its Cronbach’s alpha was measured to be 0.7; it indicated the appropriateness of the instrument (12). Based on the questionnaire guideline, total MET was calculated to categorize individuals. Accordingly, values <600, 600-3000, and <3000 MET–minutes per week represented low, moderate, and severe physical exercise, respectively (13). To explore the impact of insufficient physical activity on CVDs, high and moderate categories were integrated.
To investigate the effect of obesity on CVDs, obesity and abdominal obesity were taken into consideration. Obesity was measured using body mass index (BMI). Accordingly, individuals with BMI<30 and BMI>30 kg/m2 were considered to be non-obese and obese, respectively (14). To determine the impact of abdominal obesity on CVDs, waist-to-hip ratio (WHR) was used; values>0.95 for males and >0.90 for females were considered as abdominal obesity (15).
Blood pressure was measured using a mercury barometer. Systolic and diastolic blood pressure measurements were repeated twice on the right arm; the average of the two values was reported. Values superior to 140/90 mm Hg were considered as hypertension. Moreover, blood lipid levels were assessed and compared to the critical values (cholesterol>200 mg/dL, triglyceride (TG)>200 mg/dL, low density lipoprotein (LDL)>130 mg/dL, and high density lipoprotein (HDL)<35 mg/dL); thereby, at-risk individuals were identified.
Information about the participants’ smoking status was also gathered. People with any history of smoking (previous, daily, or occasional) were considered smokers. Also, individuals who had used hookah, drugs, or alcohol in the past were considered to be exposed to this risk factor (16).
The Organizational Ethics Committee of the Institute of Disease Research at Tehran University of Medical Sciences (http://ethics.research.ac.ir/) approved the baseline Shahedieh Cohort Study (reference: IR.TUMS. DDRI.Rec.1396.1). The approval was extended for the first follow-up (IR.SSU reference. Rec.1397.135, decision 22 January 2019).
All data analyses were carried out using R 3.4.3 software. Additionally, PAR and the related Bayesian credible interval were computed using Matrix and MCMC pack packages. It should be noted that a prior Dirichlet distribution (1, 1, 1, 1) was considered in the Bayesian method. The input data of this function were in the form of 2×2 tables. The credible interval was set at 95% (17).
This analysis was conducted on 9967 participants, including 4939 females (49.56%) and 5028 males (50.46%). The mean age of CVD individuals and healthy subjects were 57.1±8.5 and 48.2±9.4 years, respectively. The mean age was significantly higher in the patient group (P=0.001). Among the study participants, the overall prevalence was 7.95% for CVDs. Further, 7.27% of women and 8.62% of men suffered from CVDs. The main risk factor of CVDs was insufficient physical exercise. Based on the results, 65.58% of the patients and 57.17% of the healthy individuals had less physical exercise. It indicates low physical exercise in the whole population and particularly among the patients with CVDs.
Generally, in the present study, the prevalences of investigated CVD risk factors were considerable; the prevalences of obesity, abdominal obesity, hypertension, and high cholesterol in the general population were 33.87%, 53.88%, 21.25%, and 35.62%, respectively. A high percentage of missing responses was observed concerning the consumption of alcohol, smoking, hookah, and opium among female participants. Considering these missing responses, the prevalences of these factors in men were 15.39%, 52.13%, 35.18%, and 29.73%, respectively. The prevalences of the cited factors were calculated for women as follows: 0.32%, 1.26%, 4.08%, and 1.11%, respectively, which were very low among females.
The results of the Chi-square test showed a significant difference between the two genders regarding the prevalence of CVD risk factors, except for high LDL levels. Due to the observed significant differences with respect to the different prevalences of CVD risk factors among women and men, calculations were done separately for both genders.
The result of Table 1 shows that the highest PAR of CVDs (78.41%) was associated with age as a non-modifiable risk factor, followed by hypertension, abdominal obesity, physical exercise, and TG level. Therefore, despite the importance of BMI, abdominal obesity was a better CVD predictor, accounting for the considerable prevalence of CVDs.
Moreover, PAR was higher among females compared to males in most cases. It indicates that women need serious planning to improve their health status.
Table 1. Estimated PAR of CVDs main risk factors according to sex
| PAR% (Bayes 95%-CI) | ||
| Men | Women | Risk Factors |
| 7.32 (2.11, 12.89) |
15.88 (6.60, 25.21) |
Obesity |
| 31.42 (22.80, 39.92) |
46.93 (35.62, 57.50) |
Abdominal obesity |
| 14.14 (4.13, 23.77) |
25.48 (13.39, 37.39) |
Inadequate Physical Exercise |
| 40.90 (35.08, 46.55) |
49.67 (43.03, 56.16) |
High Blood Pressure |
| 0.63 (-5.24, 6.77) |
3.87 (-3.35, 11.26) |
High Cholesterol |
| 0.72 (-2.08, 3.85) |
7.14 (4.31, 10.46) |
HDL |
| 0.16 (-4.51, 5.13) |
1.17 (-3.78, 6.63) |
LDL |
| 7.18 (2.00, 12.60) |
11.98 (6.09, 18.16) |
TG |
| -11.37 (-23.58, 0.71) |
2.35 (-0.97, 8.86) |
Smoking |
| -3.03 (-6.80, 0.98) |
0.80 (-0.02, 2.09) |
Alcohol |
| -1.29 (-4.23, 2.19) |
3.13 (0.53, 6.17) |
Hookah |
| 5.96 (-0.46, 12.65) |
2.36 (0.73, 4.49) |
Drugs |
| 76.06 (68.40, 82.86) |
73.64 (65.38, 80.83) |
Age |
Discussion
This cross-sectional study aimed to determine the extent to which CVDs could be attributed to some main risk factors of disease. The data of the Shahedieh Cohort Study were used in this study; it was conducted on 9967 individuals (4939 females and 5028 males) aged 35-70 in Shahedieh, Yazd Province, Iran. Using PAR, the study aimed to determine the extent to which the disease risk could be declined in case of finding an effective method to eradicate the exposure. In this study, physical exercise, hypertension, obesity, abdominal obesity, age, high cholesterol, LDL, HDL, TG, cigarette smoking, hookah smoking and drug, and alcohol abuse were considered CVD risk factors.
Obesity is an important but preventable risk factor of CVDs. Abdominal obesity was a better predictor of CVDs compared to BMI. In an Iranian study, overweight and obesity incidence was 32.3% in a population. The PAR of this risk factor was 28.5% among females and 29.4% among males (18). In a research performed by Flegal et al., although differences were observed in the PAR of obesity regarding various factors, obesity was responsible for 5%-15% of all mortalities and 7%-44% of CVD incidence (19). The present study represented the relationship between obesity and CVDs well. Odegaard K et al. also reported 10.8%-11.7% as the PAR of obesity and overweight among patients with CVDs. He claimed that the CVD rate could be reduced (20).
Hypertension is among the main risk factors of CVDs, affecting nearly one billion individuals around the world. The prevalence of hypertension is also high in Iran, while there are low levels of control, therapeutic, and diagnostic measures (21). Tehran Glucose and Lipid Cohort Study was conducted on 5801 individuals aged above 30 years in 2011. Hypertension prevalence was found to be 27.2% among women and 23.1% among men in the cited study (22). The PARs of hypertension and laboratory serum parameters are listed in Table 1. Hypertension high PAR, introduced it as the main preventable risk factor. In this regard, Grau et al. showed that hypertension plays a major role (60%) in the incidence of CVDs. They also reported that high LDL (35%), low HDL (25%), and high cholesterol levels (20%) had effective roles in the incidence of CVDs. However, lower values were obtained regarding these risk factors in the current study (23).
Scott A Lear et al. explored the impact of physical exercise on CVDs among 130 000 individuals in 17 countries. They concluded that high physical exercise reduced the risk of CVDs in high-, moderate-, and low-income countries. Consistently, the present study findings revealed that individuals with insufficient physical activity are more prone to CVDs (24). The PAR of this risk factor was 25.48% among females and 14.14% among males. Additionally, 18.28% of the incidence of CVDs in the whole population could be attributed to insufficient physical exercise. Thus, in case an efficient preventive program enhances the physical activity level, it can be expected to decrease the incidence of CVDs by 18.28%. Evidence has also confirmed the important role of physical exercise in CVD prevention.
The current research results demonstrated low prevalences of cigarette, hookah, and drug and alcohol abuse among females. Indeed, no significant PAR was computed for these four risk factors. However, Moran et al. conducted a study in the US and reported that drug abuse contributed by 10.2% to CVD incidence (25). Takashima et al. also carried out a cohort study in Japan and showed that drug abuse contributed by 36.8% to CVD incidence (26). It should be noted that there was a high rate of missing responses regarding the aforementioned risk factors among females; only the information of 10% of the female participants was recorded. Thus, the low prevalence could be attributed to the missing responses and cultural differences of countries. Among males, the prevalences of cigarette, hookah, and drug and alcohol abuse were 52.1%, 30.2%, 29.7%, and 15.4%, respectively. A study by Kenneth J. Mukamal showed that moderate alcohol consumption has a protective effect on CVDs (27), while many other studies have shown the opposite results (28, 29). Considering the related missing responses to the four questions about cigarette, hookah, and drug and alcohol abuse, the obtained relationship cannot be trusted; since their protective effect is not significant, further precise investigations are required to introduce them as CVD risk factors.
According to our findings, age was found to be the strongest determinant of CVDs. The PAR of high-risk age was 73.64% among females and 76.06% among males. Although age is a non-modifiable risk factor, but a part can be attributed to stress, hormonal changes, and diabetes occurring in high-risk ages. Nonetheless, another part of the cited risk factor is solely associated with an increase in age; consequently, the risk attributed to CVDs cannot be controlled and prevented.
The present study had both strong points and limitations. The strong point of the study was the large sample size. However, the data related to physical exercise were collected by a questionnaire rather than calculating the metabolic score. Thus, the reported physical activities might not represent the participants’ actual physical exercise statuses.
Conclusion
CVDs are among preventable diseases with mostly preventable risk factors. Indeed, the causal relationships between CVDs and risk factors have been well identified. Considering the high prevalence of hypertension and its large PAR, it was found to be highly effective in CVD prevention. Thus, this risk factor should be taken into consideration by health policymakers. Overall, in case an effective preventive program may control CVDs by reducing the main risk factors, it can be expected to considerably reduce the incidence of mentioned diseases in the target population.
Conflicts of Interest
The authors declare no conflicts of interest
Funding and support
The Vice-Chancellor for Research of Shahid Sadoughi University of Medical Sciences financially supported the Shahedieh cohort study.
Received: 2020/06/11 | Accepted: 2020/09/15 | Published: 2020/12/31
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |