BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://journal.zums.ac.ir/article-1-6312-en.html
2- Dept. of Cardiology, Tehran University of Medical Sciences, Tehran Heart Center, Tehran, Iran
3- Dept. of Obstetrics and Gynecology, Akbar Abadi Hospital, Iran University of Medical Sciences, Tehran, Iran ,
✅ A single dose of 20 mg nifedipine tablet administered thirty minutes before IVF had no effect on improving clinical pregnancy and live birth rate. Using higher doses, or different regimens in specific patients’ subgroups may have more effect on embryo transfer success.
In- Vitro Fertilization (IVF) is a process which is initiated by stimulation of ovaries through exogenous gonadotropins, followed by oocytes retrieval under transvaginal ultrasound guidance after fertilization and embryo culture in the laboratory and transcervical transfer of embryos into the uterus1.
The first pregnancy resulting from IVF was reported in 1976.(2,3)
Despite remarkable improvements in IVF techniques, implantation rate and clinical pregnancy rate are still lower than expected follwing IVF.
The factors which affect the embryo implantation are divided into three categories of embryo quality, endometrial receptivity and transfer efficacy (4).
One of the IVF failure causes is excessive uterine contraction after embryo transfer (5,6,7). Many anti-peristaltic agents such as Ritodrine, a Beta2 agnonist8 ; Terbutaline, a Beta2 agonist9 ; Atosiban, an oxytocin antagonist (10,11) ; and Piroxicam, a Non-steroidal anti-inflammatory drug (NSAID) (12) ; and Hyoscine , an anticolinergic (13) have been experimented in various studies to improve IVF success rate with conflicting results.
Calcium channel blockers are known agents that reduce smooth muscle contractions via diminishing intracellular calcium transfer, accordingly causing uterus relaxation. Nifedipine is one of the calcium channel blockers that is utilized for treatment of preterm labor as a tocolytic in pregnancy14 whose safety has been shown over years of experience (15,16). It is also used for emergent and non-emergent hypertension during pregnancy (17,18).
Nifedipine’s common adverse effects include flushing, peripheral edema, vertigo,headache, dyspepsia, and nausea.
Because of above-mentioned properties and associated side effects and lack of sufficient knowledge, we decided to evaluate nifedipine’s effect on increasing IVF success rate before embryo transfer.
Study design
This is a double blinded randomized clinical trial (IRCT code: 20201129049532N1) that was conducted under affiliation of Iran university of medical sciences.
All the data and manuscript were reviewed and approved by ethics committee of the Iran university of Medical Sciences (Ethic code: IR.IUMS.FMD.REC.1398.556). Written informed consents were obtained from all patients.
Randomization
Randomization was performed through permuted blocks of A and B.s. Fixed block size of 6 was selected.
Patients were allocated to two groups of placebo and treatment with 1:1 ratio. Nifedipine (20 mg, Toliddaru Pharma. Co.) and placebo were administered 30 minutes before embryo transfer.
Both the patients and care givers were unaware of prescribed pills. To ensure that blindeness was as precise as possible, placebo pills were made in similar shapes to nifedipine pills. Blood pressure was measured before and after administration of nifedipine. Follow- up visits and checking serum b-hCG and transvaginal ultrasonography were performed 3 weeks after embryo transfer. Patients were followed through three- month visits if they had had successful clinical pregnancy.
Study Population:
From January 2019 to September 2019, 323 infertile women presented to Akbarabadi hospital clinic of infertility for IVF; 98 women were enrolled in the study based on patients consents, inclusion and exclusion criteria and allocated to two groups of placebo and nifedipine with 1:1 ratio, 49 patients in placebo and 49 patients in nifedipine group.
Inclusion criteria were defined as:
-
Women aged 18 to 40 years
-
Fresh or Frozen embryo transfer (ET or FET, respectively)
-
Good embryo quality (Grade A, according to cell numbers and shapes)
-
Transfer of 3 embryos on day two.
Exclusion criteria were defined as:
-
Blood pressure < 100/60,
-
Body Mass Index(BMI) > 38 kg/m2
-
Abnormal uterine cavity (congenital or acquired),
-
Nifedipine contraindications (e.g. History of severe allergic reaction to nifedipine, Porphyria, Severe Heart Failure, Significantly low blood pressure)
-
Recurrent implantation failure,
-
History of recurrent abortion.
Outcomes Definition
Primary outcome was clinical pregnancy which was defined as presence of gestational sac in the uterine cavity in transvaginal sonography three weeks after embryo transfer.
Secondary outcomes were:
1-Abortion which was defined as loss of baby before 20 weeks of pregnancy
2-Ectopic pregnancy which was defined as presence of gestational sac out of the uterine cavity in transvaginal sonography
3-Multiple gestation which was defined as presence of two or more gestational sacs in the uterine cavity in transvaginal sonography three weeks after embryo transfer
4-Live birth which was defined as number of live born neonates.
Statistical Analysis
For the sample size estimation based on primary outcome, regarding previous studies of IVF success rate which was reported about 20-25%, assuming 28% absolute increase in rate of clinical pregnancy by treatment and considering 10% attrition rate of participants, we estimated that a sample size of 98 women would be needed for a study to have a power of 80%. Data analysis was performed based on intent-to-treat follow up.
The only quantitative data in the study was the age of the patients for which Mann Whitney U test was used due to non-normal distribution of variables of the study. . Categorical variables were analyzed by chi square and logistic regression tests. Subgroup analysis was performed, by examination of interaction terms significance. Significance level was considered less than 0.05 with two-sided alpha error.
All patients were able to be followed completely. Median ages of the patients were 31 and 34 in placebo and nifedipine groups, respectively. 39 out of 48 patients (79.5%) and 31 out of 48 patients (63.2%) had primary infertility in placebo and nifedipine groups respectively (Table 1).
Table 1. Baseline Characteristics. N: Total number of patients, n: number of patients in each subgroup, IQR: Interquratile.
| P – value | Nifedipine (N = 49) |
Placebo (N = 49) |
|
| 0.266 | 34(IQR:30-37) | 31 (IQR:28-36) | Age |
| Embryo Transfer Type | |||
| 0.063 | 48.9% (n=24) | 30.6% (n=15) | ET |
| Infertility Type | |||
| 0.073 | 63.2% (n=31) | 79.5% (n=39) | Primary |
Fresh embryo transfer (ET) was performed in 15 patients (30.6%) of placebo group and 24 patients (48.9%) of nifedipine group.
Primary Outcomeand clinical pregnancy occurred in eighteen patients (36.7%) in placebo group and seventeen patients (34.7%) in nifedipine group (OR: 0.91, 95%CI (0.4-2.09)) which was not statistically significant (Table 2).
Tables 2. Table of Outcomes. N: Total number of patients, n: number of patients per treatment group.
| p-value | Odd Ratio (95% Conf. Interval) |
Nifedipine (N=49) n (%) |
Placebo (N=49) n (%) |
|
| 0.83 | 0.91 (0.40, 2.09) | 17 (34.6) | 18 (36.7) | Clinical Pregnancy |
| 0.66 | 0.82 (0.34, 1.95) | 14 (28.5) | 16 (32.6) | Live Birth |
| 0.58 | 1.71 (0.2, 11.7) | 3/17 (17.6) | 2/18 (11.1) | Multiple Gestation |
| 0.92 | 1.07 (0.22, 5.21) | 4/17 (23.5) | 4/18 (22.2) | Abortion |
Among those who had suscessful clinical pregnancy, in placebo group, two out of eighteen patients (11.1%) had multiple pregnancy and in nifedipine group, three of seventeen patients (17.6%) had multiple pregnancy(OR:1.71, 95% CI (0.2 – 11.7)).
None of the patients had multiple gestations with more than 2 fetuses.
Also, among patients who had successful clinical pregnancy, four of eighteen (22.2%) patients had abortion in placebo group and four of seventeen patients (23.5%) in nifedipine group (OR: 1.07, 95%CI (0.22 – 5.21)).
None of the patients had ectopic pregnancy.
In placebo group 2 patients had GDM (11%) and no one developed gestational hypertension.
In nifedipine group none of the patients had GDM and one patient (5.9%) developed gestational hypertension.
There was no adverse effects of nifedipine in either group.
For subgroup analysis, the patients were analyzed based on embryo transfer and infertility types. Among the patients who underwent fresh embryo transfer, seven of fiftheen patients (46.7%) (25%) and six of twenty four patients had succesful clinical pregnancy (OR: 0.38, 95%CI (0.09 – 1.5)). in placebo and nifedipine groups respectively.
Among the patients who underwent frozen embryo transfer, eleven of thirty four patients (32.3%) in placebo group and eleven of twenty five patients (44%) in nifedipine group had successful clinical pregnancy (OR: 1.64, 95%CI (0.56-477)).
In subgroups of infertility type, patients who had primary infertility, fourteen of thirty nine patients (35.9%) in placebo group and ten of thirty one patients (32.3%) in nifedipine group had clinical pregnancy (OR: 0.85, 95%CI (0.31 – 2.30)).
In secondary infertility subgroup, four of ten patients (40%) in placebo group and eight of eighteen patients (38.9%) in nifedipine group had clinical pregnancy (OR: 0.95, 95%CI (0.19 – 4.63)) (Figure 1).
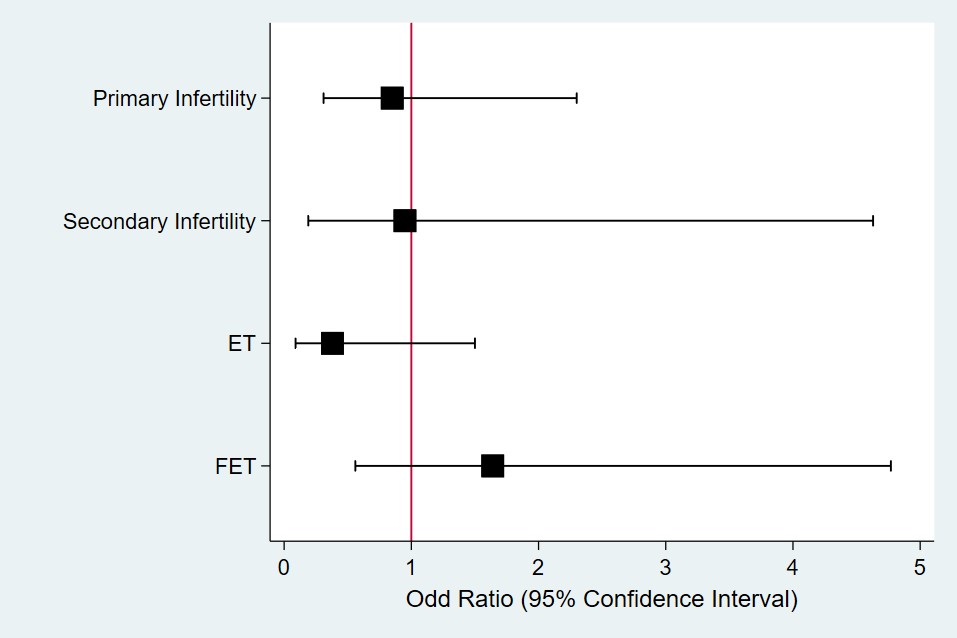
Figure 1. Subgroup Analysis Forest Plot. Odds ratio more than one favours treatment and less than one favours placebo.
In evaluation of interaction term of subgroups and treatment, there was no significant interaction between any subgroups and treatment (p value = 0.99 for Embryo type, p value = 0.62 for infertility type).
Discussion
In- vitro fertilization has become one of the main treatment modalities for infertility since 1976 (1). Many studies have confirmed its relative efficacy. But despite significant improvements in IVF techniques, its success rate is still lower than expected (19). According to literature approximately 20 – 25% of the patients who had undergone IVF, had successful implantation. Because of its relatively high cost and potential adverse effects, many attempts have been made to improve IVF results. Among them is administering drugs which increase IVF success rate. One possible mechanism of IVF failure is excessive uterine contraction during IVF process which prevents proper embryo implantation. Thus,utilization of agents that reduce uterine contraction theoritically seems to increase IVF success rate by reducing uterine contraction (6, 7). Oral short acting nifedipine, is a dihydropyridine calcium channel blocker which causes smooth muscle relaxation in varoius organs. Due to presence of calcium channel in uteirne, it has the ability to reduce uterine contraction. We conducted this double- blinded controlled trial to investigate the effect of administration of oral short acting nifedipine just before embryo transfer, on improving IVF results. In our study, nifedipine had no effect on either clinical pregnancy or live birth. Albeit not statistically significant, actually there was lower clinical pregnancy in nifedipine group. In none of the subgroups of the patients there was a difference in treatment response between placebo and nifedipine. Despite being not statisticaly significant, in nifedipine group of patients who had undergone frozen embryo transfer there was a higher clinical pregnancy. In the only study that evaluates the effect of nifedipine on IVF, nifedipine did not show any effect on IVF results (19). There is no other study assessing similar agent for IVF. The negative results of our study may be due to inadequate dose of nifedipine or inadequate estimation of sample volume. It seems that further studies with larger sample volume and/or higher dose of nifedipine are needed to assess its effect on infertility treatment by IVF.
Conclusion
A single dose of 20 mg nifedipine tablet administered thirty minutes before IVF had no effect on improving clinical pregnancy and live birth rate. Using higher dose, or different administration protocol in specific patient subgroups could have more effect on embryo transfer success.
Acknowledgements
None.
Conflicts of Interest
None declared.
Received: 2020/11/27 | Accepted: 2022/06/15 | Published: 2022/06/30
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |






