BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://journal.zums.ac.ir/article-1-6518-en.html


 , Tammy Edel Nudelman2
, Tammy Edel Nudelman2 

 , Hernando Mulett Hoyos3
, Hernando Mulett Hoyos3 

 , Alirio Bastidas4
, Alirio Bastidas4 

 , Cesar A Diaz Ritter5
, Cesar A Diaz Ritter5 

 , Jaime Fernández-Sarmiento *6
, Jaime Fernández-Sarmiento *6 


2- Dept. of Pediatric Critical Care. Fundación Cardioinfantil, Bogotá, Colombia
3- Dept. of Research, University of La Sabana (Universidad de La Sabana), Bogotá, Colombia
4- Dept. of Research, University of la Sabana, Bogotá, Colombia
5- Dept. of Pediatrics, University of La Sabana, Bogotá, Colombia
6- Dept. of Pediatrics, University of La Sabana, Bogotá, Colombia ,
✅ The use of HFNCs in children with moderate to severe bronchiolitis was associated with a modified clinical course, avoiding mechanical ventilation even in risk groups. SpO2/FiO2 and ROX index 12-hour cut-off points suggestive of patients with a delayed response to HFNC support were identified.
Bronchiolitis is the first acute wheezing episode in children under the age of two, one of the main causes of morbidity, and a cause of acute respiratory infection (ARI). Each year, there are 3.4 million hospitalizations due to bronchiolitis, most secondary to respiratory syncytial virus (RSV), and it is responsible for approximately 48,000-74,500 deaths in children under the age of five worldwide (1,2). It is the first cause of hospitalization in high-income countries in children under the age of two (1). Ten to 15% of cases are moderate to severe and require treatment in the PICU. In middle and low-income countries, treatment is largely influenced by the availability of resources and ability to access healthcare services (3).
Treatment is aimed at modifying the natural history of the disease and consists in keeping the airways patent and providing support measures: hydration, oxygen therapy to correct hypoxemia, and bronchodilators. When there is progression to respiratory failure, patients may require invasive or non-invasive mechanical ventilation (IVM or NIVM) (1,4,5). The clinical response to the measures may be unsatisfactory and the evidence regarding the efficacy of the various available treatments is still limited (4).
Ventilatory support alternatives include high-flow nasal cannulas (HFNCs) to modify the clinical course and decrease the need for IMV (6). However, even this intervention is controversial, and some centers prefer to use continuous positive airway pressure (CPAP) to avoid progression to respiratory failure (7).
The outcomes of HFNC use in patients with moderate to severe bronchiolitis are unknown, as well as the SpO2/FiO2 and ROX index values at various times after the intervention which would suggest an unsatisfactory clinical course and possible deterioration. The objective of this study was to estimate the change in the Wood-Downes scale after starting HFNC support and its relationship with unsatisfactory outcomes, as well as the change in SpO2/FiO2 and the ROX index after beginning this support in children with bronchiolitis requiring admission to the PICU.
A retrospective cohort study was carried out in the PICU of the Fundación Cardionfantil in Bogotá, Colombia between January 2013 and July 2020. This PICU is a university reference center. This study was approved by the institutional ethics and research committee with approval number DDI – 4380 – 2020. Data were taken from the electronic medical chart and recorded in a database to which only the investigators had access. When data were incomplete, the handwritten nursing notes and monitoring notes were reviewed. To avoid possible data transcription errors, the investigators double checked the clinical chart data and the Excel database, and patient information was sought in institutional laboratory, radiology and microbiology sources. The probability of data loss was reduced by guaranteeing that the data collection tool was stored with the paperwork related to the clinical chart.
Children with moderate to severe bronchiolitis (defined as the first episode of wheezing secondary to a lower respiratory tract infection in children under two years of age [1]) at any time during admission, from the emergency room to the PICU, and respiratory failure with an HFNC ordered by the attending physician, were included. Respiratory failure was defined clinically as altered consciousness and/or respiratory distress characterized by abnormal oxygen and carbon dioxide exchange in the lungs, with acute hypoxemia or hypercapnia. The patients had to have at least one PaO2 less than 60 or a PCO2 greater than 50. If the patient did not have an arterial line, the clinical criterion for respiratory failure was an SaO2/FiO2 less than 200. A diagnosis by blood gas analysis was not considered because not all patients had an arterial line, and it was important to avoid manipulation and painful stimulation that could worsen the respiratory distress. In addition, according to institutional protocols, all moderate or severe patients received systemic steroids, and adrenaline and 3% saline nebulizations. Patients who were started on mechanical ventilation prior to using HFNC were not included.
The primary variable was deterioration in the Wood-Downes scale. This scale is composed of heart rate, respiratory rate, cyanosis, wheezing, retractions, and air intake. Each parameter has a score of 0 to 4, depending on clinical findings. A score of 1 to 3 points denotes mild bronchiolitis, 4 to 7 points moderate bronchiolitis, and 8 to 14 points severe bronchiolitis. The scale has been validated and is frequently used to evaluate the severity of bronchiolitis (8). Its variables help detect changes in clinical course produced by the various interventions. However, it does not evaluate oxygenation indicators and their relationship with the respiratory rate (8).
The scale parameters were recorded and analyzed when HFNC support was begun, and at 6 and 12 hours. Other variables were also collected, including age, sex, weight, height, main caregiver, comorbidities, birth weight, oxygen saturation (SpO2), total volume of fluids, complications, length of stay in the PICU, cannula failure, SpO2/FiO2, and ROX index. This index was extrapolated from the adult description and is equivalent to SpO2/FiO2 divided by respiratory rate.
As a descriptive study, the qualitative variables were summarized in frequencies and percentages, and the quantitative variables in measures of central tendency or dispersion, according to whether they were normally distributed or not. For those with a normal distribution, means and standard deviations were used, and for non-normal distributions, medians and interquartile ranges were used. The percentage improvement is described, along with the number of subjects who changed their status. In addition, the ROX index and SpO2/FiO2 values with HFNC support were described at the given assessment times. The Youden index was then used to determine the cut-off points which were associated with worse outcomes, the need for IMV, or delayed recovery on the Wood-Downes scale. A p<0.05 was considered significant, and the SPSS 25.0 statistical package was used.
Population characteristics
During the study period, 2,390 children were admitted to the PICU with respiratory disease, 87 of whom were diagnosed with bronchiolitis and met the inclusion criteria by receiving rescue HFNC support.
The median age was 4.4 months (IQR 2.4-8.6) (Table 1). Of the patients studied, 53 (60.9%) were male, with a median weight of 6.4 kilograms (IQR 5.1-8.5) and a median height of 62 centimeters (IQR 56-69). Respiratory secretion studies were performed on 100% of cases, with respiratory syncytial virus isolated as the cause of bronchiolitis in 51%, and no isolates in 35%.
Table 1. Population Characteristics
| Total population n= 87 | Subjects with clinical score deterioration n= 11 | Subjects without clinical score deterioration n= 76 | P value | |
| Age in months Me (IQR) | 4.4 (2.4-8.6) | 7.1 (2.3-9.8) | 4.1 (2.4-8.7) | 0.57 |
| Male Sex n (%) | 53 (60.9) | 6 (54.5) | 47 (61.8) | 0.64 |
| Weight in kg Me (IQR) | 6.4 (5.1-8.5) | 6.6 (5.3-8.2) | 6.4 (5-8.5) | 0.73 |
| Height in m Me (IQR) | 0.62 (0.56-0.69) | 0.66 (0.6-0.7) | 0.61 (0.56-0.69) | 0.71 |
| Body Mass Index kg/m2 Me (IQR) | 16.3 (14.3-18) | 14.7 (13.5-16.8) | 16.4 (14.4-18) | 0.20 |
| Main Caregiver 0.55 | ||||
| Mother n (%) | 83 (95.4) | 10 (90.9) | 73 (96.1) | |
| Father n (%) | 3 (3.4) | 1 (9.1) | 2 (2.6) | |
| Grandparents n (%) | 1 (1.1) | 0 (0) | 1 (1.3) | |
| Respiratory Comorbidities n (%) |
21 (24.1) | 4 (36.4) | 17 (22.4) | 0.31 |
| Genetic Comorbidities n (%) |
6 (6.9) | 1 (9.1) | 5 (6.6) | 0.76 |
| Neurological Comorbidities n (%) |
5 (5.7) | 2 (18.2) | 3 (3.9) | 0.05 |
| Craniofacial Comorbidities n (%) |
3 (3.4) | 1 (9.1) | 2 (2.6) | 0.27 |
| Cardiovascular Comorbidities n (%) | 13 (14.9) | 4 (36.4) | 9 (11.8) | 0.03 |
| Immunodeficiency Comorbidities n (%) | 1 (1) | 0 (0) | 1 (1.3) | 0.70 |
| Prematurity n (%) | 19 (21.8) | 1 (9.1) | 18 (23.7) | 0.27 |
| Low birth weight n (%) | 21 (24-1) | 4 (36.4) | 17 (22.4) | 0.31 |
| Birth weight in gr Me (IQR) |
2,910 (2,470-3,282) | 2,730 (2,190-3,180) | 2,980 (2,550-3,288) | 0.23 |
| Breastfeeding on admission n (%) |
48 (55.2) | 5 (45.5) | 43 (56.6) | 0.77 |
| Supplementary oxygen at birth n (%) | 28 (32.2) | 6 (54.5) | 22 (28.9) | 0.09 |
Clinical progression
After starting HFNC support, 87.3% had no deterioration in their clinical severity scores. Clinical deterioration was found in 11 cases (12.6%). Altogether, 79.3% had a moderate Wood-Downes severity score on admission, and 20.7% were severe. At six hours’ follow up, 25.3% of the total population had a mild Wood-Downes score, 72.4% moderate and 2.3% severe. After 12 hours of HFNC support, the Wood-Downes score was mild in 41.4%, moderate in 55.2% and severe in 3.4%.
Out of the 87 total patients, 11 (12.6%) had clinical deterioration, as described in Table 2. At the six-hour Downe’s score follow up in this group, 27.3% had a moderate score and 18.2% had a severe score. However, at 12 hours’ follow up, the score continued to be severe in 27.3% of patients. None of those with clinical deterioration required NIMV or IMV due to respiratory failure progression.
Table 2. Clinical Outcomes and viral isolates
| Total population n= 87 | Subjects with clinical deterioration n= 11 | Subjects without clinical score deterioration n= 76 | P value | |||
| Viral isolates n (%) | 0.75 |
|||||
| Respiratory syncytial virus | 45 (51.7) | 7 (63.6) | 38 (50) | |||
| Adenovirus |
1 (1.1) | 0 (0) | 1 (1.3) | |||
| Influenza |
3 (3.4) | 0 (0) | 3 (3.9) | |||
| Negative |
31 (35.6) | 4 (36.4) | 27 (35.5) | |||
| Other | 7 (8) | 0 (0) | 7 (9.2) | |||
| Total Fluid Balance Me (IQR) | 102 (-219 - 566) | 306 (-234 - 1312) | 96 (-205 - 599) | 0.23 | ||
| Degree of dehydration on admission n (%) | <0.001 | |||||
| No Dehydration |
73 (83.9) | 6 (54.5) | 67 (88.2) | |||
| Mild |
8 (9.2) | 4 (36.4) | 4 (5.3) | |||
| Moderate |
6 (6.9) | 1 (9.1) | 5 (6.6) | |||
| Severe |
0 (0) | 0 (0) | 0 (0) | |||
| Days from onset prior to hospitalization Me (IQR) | 3 (2-4.3) | 2 (2-3) | 3 (2-5) | 0.24 | ||
| Days of hospitalization prior to PICU admission Me (IQR) | 0.82 (0-2.7) | 2.43 (0.5-3.9) | 0.77 (0-2.54) | 0.78 | ||
| Days in PICU Me (RIQ) | 3.5 (2.2-5) | 3.73 (2.6-6.4) | 3.29 (2.2-5) | 0.75 | ||
| Length of time with conventional oxygen Me (IQR) | 4 (0-30) | 28 (0-49) | 3.5 (0-28) | 0.08 | ||
| 30-day readmission n (%) | 4 (4.6) | 1 (9.1) | 3 (3.9) | 0.45 | ||
| Development of new atelectases n (%) | 15 (17.2) | 1 (9.1) | 14 (18.4) | 0.44 | ||
| Need for vasoactive support n (%) | 3 (3.4) | 3 (27.3) | 0 (0) | <0.001 | ||
In the group with clinical deterioration, the median age was 7.1 months (IQR 2.3-9.8), which was higher than the group without deterioration (4.1 months, IQR 2.4-8.7) (p=0.05). This group had a greater frequency of congenital heart disease (p=0.03), chronic respiratory disease (p=0.03) and neurological diseases (p=0.05).
With regard to clinical outcomes, detailed in Table 3, the median length of ventilatory support with HFNC was 3.5 days (IQR 2.2-5). There were no differences in length of HFNC support between those who did not deteriorate and those who did. No patients had failed HFNC (need for orotracheal intubation, NIMV, progression to ARDS and/or progression to multiple organ failure). The patients whose Wood-Downes severity scores deteriorated had a greater need for vasoactive support (p<0.001).
With regard to fluid balance, the cumulative balance in the HFNC group with a worsening clinical severity score was 4.6%, compared with a 1.3% cumulative fluid balance in the group that did not worsen.
The SpO2/FiO2 cut-off points were identified using the Youden index, evaluating non-deterioration of the Wood-Downes severity scale. At the onset of HFNC support, the cut-off point was 184, with a sensitivity of 51% and a specificity of 64% (PPV 17% and NPV 90%). At 6 hours, the cut-off point was 157, with 93% sensitivity and 27% specificity (PPV 16% and NPV 97%). At 12 hours, the cut-off point was 180, with 95% sensitivity and 27% specificity (PPV 16% and NPV 97%) Figure 1.
The ROX index value found using the Youden index, evaluating worsening of the Wood-Downes severity scale, was 3.8 on admission, with 61% sensitivity and 55% specificity (PPV 16% and NPV 91%). At 6 hours it was 4.6, with 66% sensitivity and 34% specificity (PPV 21% and NPV 93%). At 12 hours, the value was 4, with 91% sensitivity and 45% specificity (PPV 19% and NPV 97%) (Figure 2). In other words, if patients have a ROX index greater than 4 at 12 hours, 91% of them will not have clinical deterioration measured with the Wood-Downes severity scale.
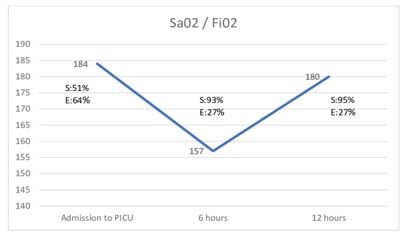 |
Figures 1. 1.Sao2/Fio2 at the time of evaluation. |
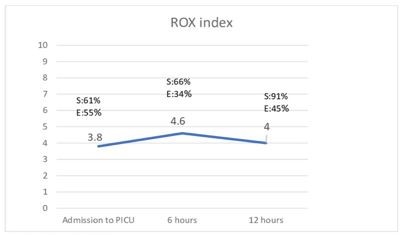 |
Figures 2. Rox INDEX at the time of evaluation. |
S: Sensitiviy. E: Specificity
Discussion
This study found that 87% of the patients with moderate to severe acute bronchiolitis who require admission to intensive care and receive HFNC support have a good progression, with no deterioration of the Wood-Downes clinical severity score. The 12.6% who had deterioration were heart disease, chronic respiratory disease and neurological disease patients. However, none of the patients required more advanced support such as NIMV or IMV. In addition, an SpO2/FiO2 less than 180 or an ROX index less than 4.0 at 12 hours were found to be good predictors of patients with a delayed response to rescue therapy with HFNC.
To the authors’ knowledge, no articles were found in the literature on the progression of the Wood-Downes severity score and outcomes with the use of HFNC in children with moderate to severe bronchiolitis. Vásques-Hoyos et al. sought to identify factors associated with HFNC failure in children with and without bronchiolitis. They found that comorbidities such as prematurity, low birth weight, heart disease, and neurological or chronic respiratory diseases were predictors of HFNC support failure (9). In addition, in Morosini et al.’s study, patients with cardiorespiratory, genetic and neurological comorbidities were associated with worse outcomes and the need for invasive ventilatory support (10). Our study had similar findings, but only in patients with moderate to severe acute bronchiolitis.
On the Wood-Downes severity scale, most had moderate to severe involvement. In patients using HFNC, none died or required NIMV or IMV. Sztrymf et al. and Frat et al. found a similar response with HFNC, such as reduced respiratory rate and IMV in patients with acute respiratory failure, along with improved oxygenation and handling of respiratory secretions (11,12). In addition, HFNC could improve hypoxemia due to less dilution of the oxygen with room air; improved gas exchange through decreased dead space and its CPAP-like effect; humidification of the system (which achieves better oxygen tolerance, decreasing respiratory effort and the metabolic cost of gas conditioning); and facilitation of mucus expulsion (decreasing atelectasis and airway resistance thanks to improved mucocilary function).
Despite the comorbidities and severity of the clinical picture as measured by the Wood-Downes scale, the lengths of stay in the PICU were similar. Franklin et al. and Riese et al. found that the use of this therapy did not produce significant changes in terms of hospital and PICU stay, the need for IMV or 30-day readmission (13,14). Furthermore, Morosini et al., in a retrospective descriptive study in Latin America, had similar findings, reporting that this therapy is effective in terms of decreasing associated complications. However, it is important to clarify that this study included more diseases which cause acute respiratory failure (10).
Our study identified an SpO2/FiO2 cut-off point for each assessment point. The three cut-off points were shown to be sensitive for lack of deterioration on the Wood-Downes severity scale and to have an NPV greater than 90% for predicting deterioration on the scale. No articles were found in the literature which specified an SpO2/FiO2 cut-off point in children or adults as a predictor of delayed response to the treatment. In our experience, an SpO2/FiO2 less than 180 at 12 hours has a 95% sensitivity for predicting delayed response to HFNC.
No studies were found evaluating the ROX index in pediatric patients. Roca et al.’s study evaluates the ROX index as a predictor of HFNC success in adults with hypoxemic respiratory failure secondary to pneumonia, finding that levels less than 3, 3.5 or 4 at one hour, six hours or 12 hours predict the need for intubation, and a value greater than 4.88 is a determinant of success in these patients (15). In our study, a value greater than 4.0 at 12 hours was also a good predictor of good progression in children with bronchiolitis receiving HFNC support.
We consider that our study has some limitations. First, it is the experience of a single center which cares for highly complex patients, and does not have a comparison group with another type of oxygen therapy. This limits our findings to the population of patients with bronchiolitis who receive HFNC support. In addition, as it is a retrospective study, there could be information bias, for which we tried to control by reviewing not just the primary information source recorded in the electronic clinical chart, but also the digital and manual data from the nursing notes. The observational nature of the study allows hypotheses to be made regarding the role of HFNC in modifying the natural course of the disease, which should be confirmed in controlled clinical trials.
Conclusion
The use of rescue HFNC in children with moderate to severe bronchiolitis was associated with a modified clinical course of the disease, avoiding progression to acute respiratory failure which would require invasive or non-invasive MV. Twelve-hour cut-off points for SpO2/FiO2 and the ROX index were identified as indicators of patients with delayed response to this treatment, but who nevertheless do not require more advanced measures for managing respiratory failure.
Acknowledgements
We would like to thank the medical, nursing and respiratory therapy team of the Pediatric ICU for their kind cooperation, as well as the clinical laboratory of the Fundación Cardioinfantil-IC and all the institutions which participated in patient inclusion.
Conflicts of Interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Received: 2021/04/24 | Accepted: 2021/10/11 | Published: 2022/06/30
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |



