BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://journal.zums.ac.ir/article-1-6867-en.html


 , Seyed Hojjat Hosseini2
, Seyed Hojjat Hosseini2 

 , Elham Ahmadian3
, Elham Ahmadian3 

 , Mohammadreza Ardalan3
, Mohammadreza Ardalan3 

 , Koorosh Kamali4
, Koorosh Kamali4 

 , Sepideh Zununi Vahed3
, Sepideh Zununi Vahed3 

 , Saeed Sardari1
, Saeed Sardari1 

 , Narjes Khavasi
, Narjes Khavasi 

 5
5
2- Dept. of Pharmacology, School of Medicine, Zanjan University of Medical Sciences, Zanjan, Iran
3- Kidney Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
4- Dept. of Public Health, School of Public Health, Zanjan University of Medical Sciences, Zanjan, Iran
5- Dept. of Persian Medicine, School of Medicine, Zanjan University of Medical Sciences, Zanjan, Iran , nxavasi@zums.ac.ir
✅ Post-CSSE (50 and 100 mg/kg) treatment could protect against cisplatin-induced nephrotoxicity in vivo. More clinical studies are needed to confirm its protective effects on the prevention of kidney injury in chemotherapy receiving patients.
Acute kidney injury (AKI) is a common problem in many clinical conditions including cancer (1-3). Cancer chemotherapy is also associated with several side effects including AKI (4). Cisplatin is an important chemotherapeutic drug that is commonly used in cancer therapy protocols for solid tumours such as head and neck, ovary and testis, breast and lung (1, 5). A single-dose of Cisplatin can induce nephrotoxicity in one out of three patients undergoing chemotherapy, which can be increased by repeated doses (4). Cisplatin accumulates in the proximal tubules of the kidney, with a concentration of five times higher than the serum concentration, leading to nephrotoxicity (4). Cisplatin induces renal damage via inflammation, oxidative stress, apoptosis and necrosis (6).
The use of natural compounds that can protect against chemotherapy-induced organ injuries has grabbed great interest. World Health Organization reported that 80% of the world population mainly use traditional medicine (7). Capparis genus (in the Capparaceae family) contains more than 250 species many of which are grown in the Mediterranean region and the Middle East. Capparis spinosa is a common type of Capparis genus that grows in the Mediterranean region (8, 9). Capparis spinosa is an ancient medicinal plant that has been used as a therapeutic agent in the traditional medicine of many nations (8). It contains high amounts of bioactive antioxidant components (8, 10) and some studies suggest that its leaves and seeds could enhance kidney function (11, 12). Plenty of experimental studies have evaluated the possible beneficial effects of different parts of this plant (8, 13-19). However, there are limited studies on the possible protective effects, particularly nephroprotection, of the plant’s seeds (11). The aim of our study was the evaluation of the nephroprotective effect of seedextract of Capparis spinosa on kidney histopathological changes following cisplatin-induced AKI.
Popularity and growing interest in complementary and traditional medicine and natural products (7, 8) motivated us to investigate the protective effect of an important plant in Persian Medicine, Capparis spinosa, against nephrotoxicity induced by cisplatin in vivo.
Chemicals
Cisplatin vials for intraperitoneal injection were prepared from the Mylan (France) 50mg/50 ml. Ethanol 96% was prepared from SIMIN TAAK.CO. (Zanjan-Iran) for ext. preparation.
Seed extract
Seeds of Caparis spinosa fruit were collected from the Moghan province of Iran. Shahid Beheshti’s pharmacology faculty lab herbarium confirmed the species of plant. Seeds of fruit were extracted and dried in shade. Seeds were powdered by grinder (KEEP grinder model KG-250 Made in Korea) and then dissolved in ethanol/water at a 70/30 ratio, and preserved at room temperature for 48 hours. Then the extracted fluid was dried and the extract was obtained. These steps were repeated three times for the whole extraction.
Experimental animals
Before starting the study, the approval for the animal study was obtained from the ethics committee of Zanjan University of Medical Sciences (IR.ZUMS.REC.1399.402). Forty male Sprague Dawley rats weighing 230±20 gr were randomly divided into 8 groups. Animals were prepared from the Pasture Institute (Tehran- Iran) and maintained in a standard situation (12 hours light/darkcycle, 22 °C, and free access to water and food).
Experiment
The studied animal groups were as follows: A: sham, B : Cisplatin 7mg/kg intraperitoneal (IP),
C : Capparis Spinosa seedhydroalcoholic extract (CSSE) 200 mg/kg BID orally for 12 days (as toxic dose), D : Cisplatin 7 mg/kg (IP) single dose+ CSSE 50 mg/kg BID for 12 days, E : Cisplatin 7 mg/kg single dose (IP)+ CSSE 100 mg/kg BID for 12 days, F : Cisplatin 7 mg/kg single dose (IP) +CSSE 50 mg/kg BID for one day, G : Cisplatin 7 mg/kg single dose (IP) +CSSE 100 mg/kg BID for one day, and H : CSSE 100 mg/kg pre-treatment for 12 days then Cisplatin 7 mg /kg single dose (IP). All doses of extract were administered by gavage.
Forty-eight hours after the end of interventions, sampling was done and left kidneys of animals were extracted after rats were anesthetized by ether.
Histopathological examinations
The lower half of the left kidney tissue of rats was placed in 10% formalin /normal saline solution at room temperature. The solution was exchanged with a new fresh solution after 12 hours of sampling. Samples were transferred to the laboratory and paraffinated samples were prepared. Then, samples were deparaffinized and stained with hematoxylin and eosin (H and E). Two categories of the histopathological changes (glomerular and tubular changes) were studied. Infiltration of inflammatory cells in the kidney tissue of rats was also studied. Microscope model was BM-600 LED EPI FLURESCENT Germany-AXIOM and Camera model was Mshot made by China.
Statistical Analysis
For quantification and analysis of pathological changes also to diminish human errors in analysis, Image J Macro Language (IJM) program 1.52n (20-22) was used. Then, Graf Pad Prism software was used for analyzing quantified data. Data were checked by Shapiro-Wilk normality test and all data showed a normal distribution. One-way ANOVA test was used for analyzing quantified pathological changes in the kidney tissue in groups of study, followed by Tukeys post-hoc multiple comparison test.
Histopathology
Glomerular alterations in Bowman’s capsule area, bowman's space, inflammatory cells, and tubular injury including tubular dilatation, loss of brush borders, tubular cast formation, and tubular necrosis were evaluated by Hematoxylin and eosin (H and E) staining of kidney tissues and Image –J program (20-22) (Figures1 & 2). All groups were compared with control and cisplatin groups (Figures 1 & 2).
Glomerular changes
Glomerular area
Normal glomerulus was exhibited in kidney cortex of the control group. Shrinkage of glomeruli and diminution of the glomerular area were seen in the cisplatin group and CSSE 200 mg/kg -receiving group compared to the control group (p< 0.0001 and p< 0.0002, respectively). Treatment with CSSE 50 and 100mg/kg after cisplatin injection for 12 days and one day administration of 100mg/kg of CSSE after cisplatin significantly prevented glomerular shrinkage, respectively, p< 0.0006, p< 0.005, p< 0.01, in (Figure 1).
Bowman's capsule area
Bowman's capsule area in cisplatin group was lower than the control group p< 0.01. All CSSE -receiving groups non-significantly reversed it to normal range except one group that was treated one day by 50mg/kg CSSE after cisplatin injection (Figure 1).
Bowman's space
Bowman's space (urinary space) in cisplatin-injected group in comparison with control was increased (p< 0.0001) which was reversed in all treated groups with CSSE significantly except the pretreated with CSSE group (Figure1).
Inflammation
Inflammatory cells in the cortex of kidney were also assessed by image-j, and it was shown that inflammatory cells infiltration occurred in cisplatin-receiving group compared to the control group (p< 0.0001). This infiltration was diminished in groups that received CSSE 50 (p< 0.003) and 100 mg (p< 0.04) for 12 days after cisplatin injection.
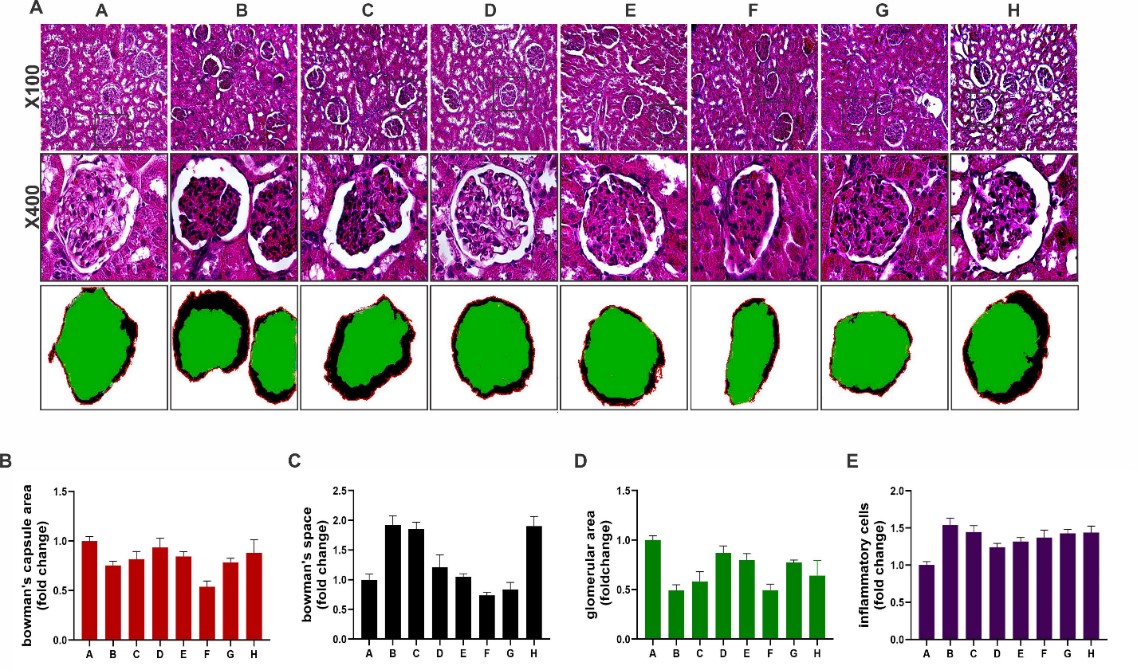
Figure 1. Kidney glomerular histopathological changes in the studied groups. A: Effects of cisplatin and Capparis spinosa seed hydroalcoholic extract (CSSE) treatment on kidney glomerular histopathological changes in hematoxylin and eosin (H and E) stained kidney slides by magnification x100 and x200 were scored and analyzed by image J program and one way ANOVA test and tukey᾿s multicamparson tests between groups of study and p<0.05 was considered statistically confident. Groups of study: A:sham, B: cisplatin 7mg/kg intraperitoneal(IP)single dose, C : Capparis spinosa seeds hydroalcoholic extract (CSSE) 200 mg/kg BID orally for 12 days (as toxic dose), D : Cisplatin 7 mg/kg (IP) single dose then CSSE 50 mg/kg BID for 12 days, E : Cisplatin 7 mg/kg single dose (IP) then CSSE 100 mg/kg BID for 12 days, F : Cisplatin 7 mg/kg single dose (IP) then CSSE 50 mg/kg BID for one day, G : Cisplatin 7 mg/kg single dose (IP) fallowed CSSE 100 mg/kg BID for one day, H : CSSE 100 mg/kg pre-treatment for 12 days then Cisplatin 7 mg /kg single dose (IP) day 12th All doses of extract administered by gavage). B: Bowman's capsule area changes; cisplatin group versus the control group (p< 0.01). C: Bowman's space (urinary space); cisplatin group versus control (p< 0.0001) reversed all treatment groups significantly except group H. D: Shrinkage of glomeruli; cisplatin group and CSSE 200 mg/kg versus control (p< 0.0001) that was reversed by CSSE 50 and 100mg/kg for 12 days and 100mg/kg of CSSE one day significantly p< 0.0006, p<0.005 and p<0.01 respectively. E: inflammation; cisplatin group and CSSE 200 mg/kg versus control p<0.0001. Inflammation was reversed by CSSE 50 and 100mg/kg for 12 days and 100mg/kg of CSSE one day significantly p< 0.0006, p<0.005 and p<0.01 respectively.
In other treatment groups, the inflammatory cells were also plunged but the results were not statistically significant. In the group that received only high dose CSSE 200mg/kg, inflammation was seen compared to sham (p< 0.0001,Figure 1).
Tubular injuries
Tubular loss of brush borders
Tubular loss of brush borders was observed in cisplatin-injected group, and CSSE 200mg/kg -receiving group compared to the control group (p<0.0001). These changes were reversed in treatment groups by CSSE 50 mg for 12 days after cisplatin injection and CSSE 50 and 100mg/kg for one day after cisplatin p< 0.0001 and in the group that received CSSE 100mg /kg after cisplatin injection p< 0.02.
Tubular necrosis
Tubular necrosis was seen in cisplatin group and CSSE 200 mg/kg receiving group compared to the sham (p< 0.0001). Tubular necrosis was improved significantly in groups that were treated by CSSE 50, 100 mg/kg for 12 days and groups treated by 50 , 100 mg/kg for one day after cisplatin injection p<0.0001. In the group pre-treated by CSSE 100 mg/kg that then received cisplatin, healing was not significant (p< 0.8).
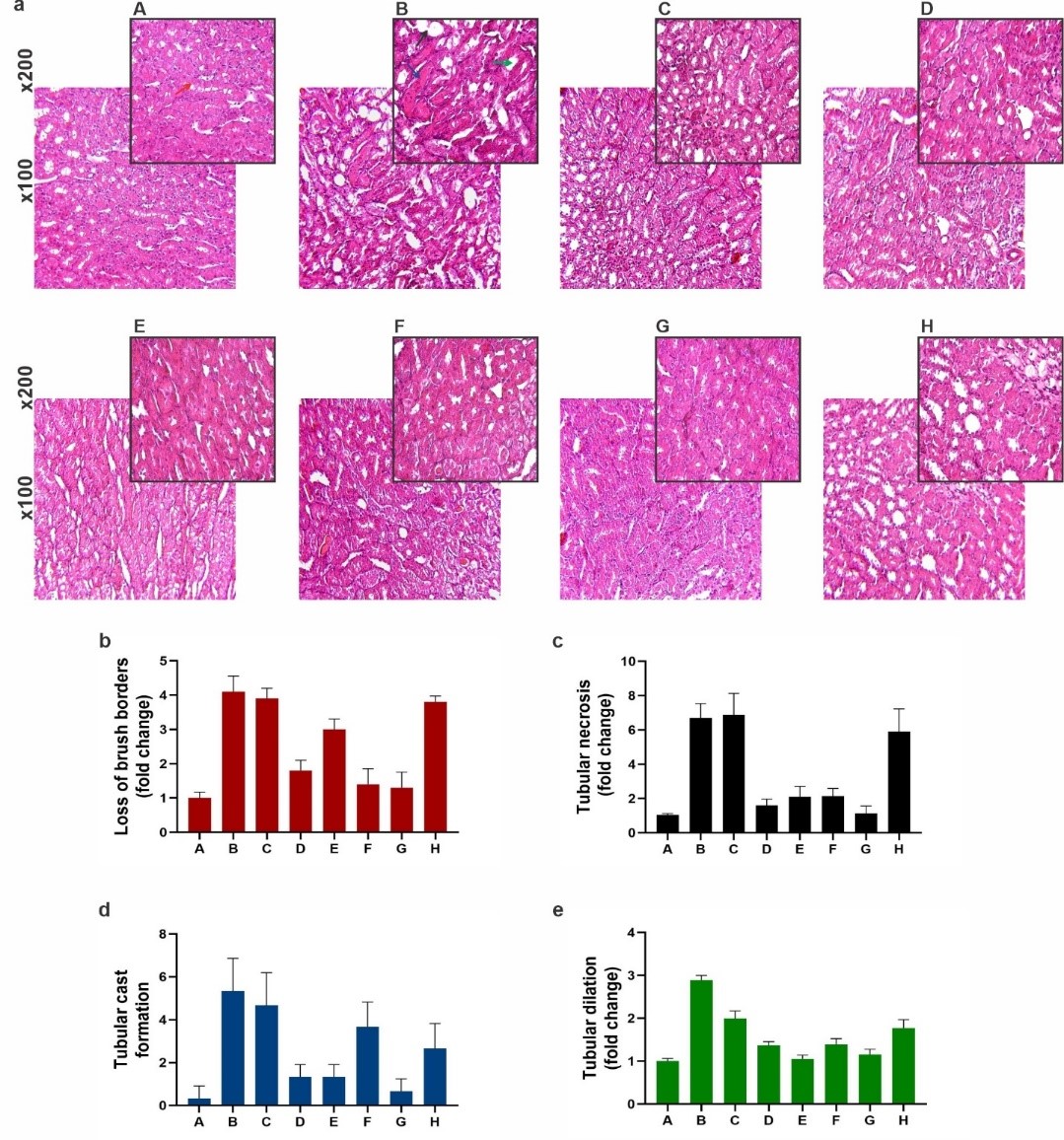
Figure 2. Kidney tubules histopathological changes in the studied groups. a: Effects of cisplatin and Capparis spinosa seed hydroalcoholic extract (CSSE) treatment on kidney tubular histophatological changes in Hematoxylin and Eosin (H and E) stained kidney slides by magnification x100 and x200 were scored and analyzed by image J program and one way ANOVA test and tukey᾿s multicamparson tests between groups of study and p<0.05 was considered statistically confident. Groups of study: (A:sham, B: cisplatin 7mg/kg intraperitonealy(IP)single dose, C : Capparis spinosa seed hydroalcoholic extract (CSSE) 200 mg/kg BID orally for 12 days (as toxic dose), D : Cisplatin 7 mg/kg (IP) single dose then CSSE 50 mg/kg BID for 12 days, E : Cisplatin 7 mg/kg single dose (IP) then CSSE 100 mg/kg BID for 12 days, F : Cisplatin 7 mg/kg single dose (IP) then CSSE 50 mg/kg BID for one day, G : Cisplatin 7 mg/kg single dose (IP) fallowed by CSSE 100 mg/kg BID for one day, H : CSSE 100 mg/kg pre-treatment for 12 days then Cisplatin 7 mg /kg single dose (IP) day 12th (All doses of extract were administered by gavage). b: Tubular loss of brush borders; cisplatin group and CSSE 200 mg/kg versus control (p < 0.0001) that were reversed by CSSE 50 mg for 12 days and CSSE 50 and 100mg/kg for one day p< 0.0001 , by CSSE 100mg /kg for 12 days p< 0.02. c: Tubular necrosis; cisplatin group and CSSE 200 mg/kg group versus sham p< 0.0001 reversed CSSE 50 , 100mg/kg for 12 days and 50 , 100 mg/kg for one day after p< 0.0001. d: Tubular cast formation; cisplatin group and CSSE 200mg/kg group versus sham p< 0.0005 and p< 0.002 respectively reversed by CSSE 50 , 100 mg/kg for 12 days p<0.004 and by CSSE 50 , 100 mg/kg one day p<0.001 and p< 0.09 respectively. e: Tubular dilatation; cisplatin and CSSE 200mg/kg groups versus sham p< 0.0001 reversed by CSSE in all groups significantly p< 0.0001.
Tubular cast formation
Tubular cast formation occurred in cisplatin group and CSSE 200mg/kg receiving group compared to sham (p< 0.0005 and p< 0.002 respectively).
Treatment with CSSE in all groupsthat had received cisplatin could prevent tubular cast formation substantially and in groups that were post- treated by 50, 100 mg/kg CSSE for 12 days p<0.0045, in groups treated by 50, 100 mg/kg one day p< 0.001 and p< 0.09 respectively and in animals pre-treated by 100mg/kg with CSSE for 12 days (p< 0.09).
Tubular dilatation
Tubular dilatation occurred in cisplatin-administered group and in rats of group that had received high dose CSSE (200mg/kg) (p< 0.0001).Treatment by CSSE in all groups could reverse cisplatin’s toxic effect significantly (p< 0.0001)(Figure 2).
Discussion
In this study, CSSE administration (doses 50 and 100 mg/kg) after cisplatin injection could present renoprotective effects against cisplatin-induced histopathological changes. However, pre-treatment with CSSE failed to show positive effects.
Cisplatin is an important drug in many cancer chemotherapy protocols but nephrotoxicity of this drug causes limitations in patients’ treatment (4). Cisplatin causes mitochondrial injury because of production of reactive oxygen species. In cisplatin-induced renal toxicity, tubular cell injury is the major pathological finding although glomerular injuries were also observed (1, 2). The accumulation of cisplatin in the proximal tubules leads to kidney toxicity, tubular injury, and cell death in a dose and time- dependent manner (1). Cisplatin is converted metabolically to toxins that cause DNA and mitochondrial DNA injury, and cell respiration damage by activation of apoptotic pathways, and induction of inflammatory responses (4). Kidney function failure and increment of serum creatinine, urea and uric acid as well as oxidative stress have been observed after cisplatin administration (4, 11, 23).
The previous report has shown anti-oxidative and anti-inflammatory effects and renoprotective effects of Capparis spinosa (11). These activities of Capparis spinosa are supported by ancient therapeutic effects of this plant (11, 24, 25). In our study we used the best antioxidative product of Capparis spinosa seed hydroalcoholic extract, which was confirmed by a pilot study.
Glomerular and tubular injuries after cisplatin administration have been reported previously (2, 26, 27). E. Yulug et al., have shown glomerular and tubular injures of cisplatin such as degeneration of glomerular structure, dilatation of Bowman’s space, thickening of the parietal layer of Bowman’s capsule and the basal membranes of tubules, tubule degeneration, loss of brush borders, and tubule dilatation (27). In our study, histopathological findings about glomerular and tubular injuries by cisplatin were seen in kidney tissue samples including shrinkage of glomeruli and decrease in glomerular and bowman's capsule area, increase in bowman's space as well as tubular changes including tubular loss of brush borders, tubular dilatation, tubular cast formation, tubular necrosis, and the infiltration of inflammatory cells that were all reversed in pre-treated groups with CSSE (2, 12). Tir et al., reported anti-fibrosis effect of pre and post treatment of CSSE in kidney of cisplatin-receiving animals (11). In another study conducted by Tilli et al., it was shown that cisplatin induces tubular atrophy, enlarged interstitial space, multiple foci of hemorrhage, inflammatory leukocyte infiltrations and dilatation of proximal tubule in vivo, where pre and post treatment by Capparis spinosa leaveextract could reverse these detrimental effects (12).
Many analytical studies have been carried out on the ingredients of different parts of Capparis spinosa (8, 28) and their antioxidative and anti-inflammatory properties (8, 10, 11, 14, 16, 29-31). Recent analytical studies on seeds of Capparis spinosa indicate that its main constituents are glycosinolates ingredients, and glucocapparine that are derived by chromatography method (8, 32). Oil amount of seeds is high (about 30%) (32), and contains mostly linoleic acid and to a lesser extent oleic acid, linolenic and other saturated and unsaturated fatty acids (32). Miristic acid that is a rare and valuable fatty acid is found in Capparis spinosa seeds (32). Other important components of seeds are tocopherols including γ-tocopherol and δ-tocopherols that are components of vitamin –E (33).
In this study, post-cisplatin administration of CSSE (doses 50 and 100 mg/kg) presented good kidney protective effects against histopathological changes. Likewise, the extracts of other parts of the plant had good protective effects on kidney, liver and some other organs pre and post cisplatin treatment (11, 34). However, pre-treatment with CSSE failed to show positive effects. Pre-treatment with CSSE has not been investigated by this seed extract. In a study conducted by Tir et al., administration of seed extract 7 days before oral administration of cisplatin, was reported to exhibit kidney protective effects (11). However, more studies are needed to investigate the mechanisms involved in the nephroprotective effects of Caparis spinosa.
High doses of Capparis spinosa can induce toxicity in the kidney and liver tissues (35). We found that CSSE 200mg/kg for 12 days in vivo had slightly malicious effects on kidney tissue pathologically. More studies are needed to find out the underlying toxic mechanisms.
Conclusion
Post-CSSE administration (doses 50 and 100 mg/kg) could significantly preserve kidney histology against cisplatin-induced AKI and indicate renoprotective effects. Due to the significant protective effects of Capparis spinosa seeds hydroalcoholic extract in our study in the therapeutic doses, clinical trials are recommended. This natural product with kidney protective effects could be used in cancer patients that are under cisplatin-therapy and would save kidney capacity for better therapy and life quality. However, more studies are required to elucidate its mechanism of action.
The main limitation of our study was the use of whole seed extract. It is suggested that future studies determine the active component of the seed extract responsible for this protection. However, the use of Image-J software and diminishing human errors in evaluation of pathological changes were the strengths of our study.
Acknowledgements
This research was financially supported by Zanjan University of Medical Sciences, Zanjan, Iran (Grant number #4116).
Conflicts of Interest
None.
Received: 2022/01/27 | Accepted: 2022/05/10 | Published: 2022/12/12
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |



