BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://journal.zums.ac.ir/article-1-6928-en.html
2- Nursing and Midwifery Care Research Center, Mashhad University of Medical Sciences, Mashhad, Iran
3- Dept. of Social Medicine, Faculty of Medicine, Sabzevar University of Medical Sciences, Iran ,
✅ The results of this meta-analysis demonstrate that intraoperative interventions, such as Peritoneal Suction Drainage, injection of Intraperitoneal Hydrocortisone with Bupivacaine, and warm and humidified insufflation carbon dioxide, can reduce the severity of SP in patients with LS. In addition, clinical trials with different interventions are needed to compare the efficacy and find effective interventions for SP management in patients with LS.
Laparoscopic surgery (LS) is nowadays broadly acknowledged as a type of operation with minimal invasion and has replaced the traditional laparotomy over time (1). Despite the improvement of patient consent with LS, a large number of patients complain of pains after surgery (2). For this reason, LS is often ascribed to operations in surgery, such as intraperitoneal insufflation of carbon dioxide (co2), leading to peritoneal stretching, diaphragmatic irritation, alterations in intra-abdominal (power of hydrogen) pH, and containment of the insufflated gas inside the abdominal cavity following the operation (3).
The pain felt by a patient following LS is dividable into three forms: parietal, visceral, and shoulder pain (SP), which differ in intensity. Whereas parietal and visceral pains lessen after twenty four to forty eight hours, SP is probably of greater significance (4). SP is one of the unpleasant postsurgical symptoms (5) that has been found in up to 2/3 of patients (6) and its severity rises constantly in the first three to six hours after surgery, reaching the utmost intensity 12 hours post-operation (7).
In different studies, such as Imbelloni (2014), Kalaivani (2014), and Ko-iam (2016) the frequencies of SP varied from 27 to 80% (8-10). By definition, SP is a sense of pain in the shoulder (11). Following laparoscopic cholecystectomy (LC), SP may represent emotional indications and alterations in vital symptoms, vomiting and nausea.Inpatients with SP, pain may be indicated as nauseous, sharp, deep and boring (5).
Despite this, the severity of postsurgical abdominal and SP is still important, restricting LC as a same-day method in great number of patients (12).
Post-surgical abdominal and SP after LC is the leading causes of anxiety to patients, though with less severity than that of an open method (13).
The precise mechanisms of SP following LS are not known (11). Apparently, they have a multifarious mode of action, but it is mostly theorized to be CO2 gas retention among the hepatic dome and right diaphragm (14). Other suggested reasons are neuropraxia from the phrenic nerve, elevated stretch on the diaphragmatic attachments of the liver, stretching of the subdiaphragmatic fibers due to the pneumoperitoneum, caused by losing visceral surface tension and peritoneal injury of ischemic, chemical, or traumatic hurt (4).
Taş (2013) and Suginami (2013) believe that post-surgical SP is assumed to stem from pneumoperitoneum attained by CO2 insufflation and remaining in the abdomen, thereby inducing peritoneal stretching and irritation of the phrenic nerve and diaphragm , leading to inflicting pain to the shoulder (1, 2).
Cha (2012) states that shoulder tip pain is caused by diaphragmatic stretching with phrenic nerve neuropraxia (6). Singh (2013) denotes that SP following LC happens upon turning CO2 into carbonic acid on the peritoneal surfaces. SP is caused by peritoneal insufflation, particularly with the use of an exaggerated trendelenburg position. Using the trendelenburg position in the long run is a cause of SP as well (11).
In a number of investigations, the relief of shoulder tip pain in laparoscopy has been assessed by various methods (15, 16). The focus of some investigations was on operation practices, pressure, temperature, and moisture of pneumoperitoneum, drainage, and various practices to alleviate the phrenic nerve stimulation (2, 17-19). In other investigations, drugs were administered to evaluate SP prevention (20-25). Alkhamesi (2007) demonstrates that local anesthetic methods are a portion of the multifaceted strategy for postsurgical pain control (15); however, the findings of the investigations require careful examination.
Therefore, postsurgical SP is a typical unwanted consequence following laparoscopic practices because LS is highly prevalent. SP emerges mostly following patient verticalization and may persist even following standard prescription of analgesics (14).
However, various techniques to reduce SP include drainage, pulmonary recruitment maneuver (PRM), preoperative premedication with drugs, drainage, positioning, low-pressure carbon dioxide, instillation of drug and fluid into the peritoneal cavity, acupuncture treatments, neuromuscular blockade, etc. (1,5,6,11,12,26-28); however, several studies with various findings have been conducted in this regard.
Regarding the different results of these interventions in mitigating SP following LS, it is necessary to conduct a meta-analysis that provides abvious and consistent results and suggestions for researchers. Overall, systematic review and meta-analysis are essential tools for summarizing available evidence reliably and accurately.
Thus, this study attempted to identify and assess the interventions used to reduce shoulder pain following LS.
Search strategy
The current systematic review aims to examine the interventions to mitigate laparoscopy-induced SP using the standardized instruction of systematic review (PRISMA), consisting of 27 items (29). All English articles from 2009 to 2020 that were relevant to our research were included in this study.
The review was implemented through researching electronic data banks, namely ISI Web of Science, PubMed, MEDLINE, Cochrane, ProQuest, Scopus, Google Scholar, and Scientific Islamic Database (SID. Medical Subject Headings (MESH) of Laparoscopic Surgical Procedures, Surgery Laparoscopic, Laparoscopic Assisted, Shoulder Pain, Pain, Shoulder, randomized controlled trial, clinical trial, and Boolean operators of "OR" and "AND" were searched for qualified studies.
Selection criteria
Abstracts were extracted and reviewed from all articles. Using the inclusion criteria and exclusion criteria, unrelated topics were omitted and relevant articles were selected for the study. The entry criteria of articles were those published in reliable scientific research journals, articles in English, and full text of papers. LS consists of LC, appendectomy, extra peritoneal inguinal hernia repair, and gynecologic laparoscopy.
Exclusion criteria were all the studies reported in congresses, conferences, with no available full-text, observational and descriptive studies, case series, reviews and letters to the editor, reported information in the form a new article, and those with incomplete results.
Data extraction
Data was extracted based on the author’s name, publication year, article type, measuring tools, sample size, results and information relevant to “Laparoscopies Surgery”, “Shoulder Pain”, “randomized controlled trial”, “clinical trial”, and final results of papers. The quality of included studies in the systematic review was examined by 2 independent evaluators. The articles were analyzed using a researcher-made data extraction form according to the research goals. In the current research, the key questions were: “As reported in the literature, what are the interventions used to mitigate SP following LS? And which of the interventions are most efficacious in mitigating SP following LS?
Statistical methods
The standardized mean difference (SMD) was used as a measure of associations between random-effect meta-analysis performed on studies evaluating the intervention group, and fixed-effect meta-analysis on studies investigating other cases.
This analysis was restricted to control trials that evaluated the relationship between standard mean differences by fixed- and random-effects models. The heterogeneity of the articles was specified using the I2 index. I2 < 0.25, 0.25 < I2 < 0.75, and I2 > 0.75 were indicators of low, average, and high heterogeneity respectively. To analyze the combination of the results of studies, the analysis was “meta” command and was performed using the Stata V12 software. P-values < 0.05 were taken to be significantly different. Heterogeneity was verified by Chi-squared based on Q-test and I2 statistics.
Totally, 93 primary articles were retrieved in the initial literature search. Among these, 44 duplicate and irrelevant articles were removed after considering the exclusion and inclusion criteria and finally, 39 full-text articles were systematically reviewed. The flowchart of the study reviews is shown in Figure 1. All studies were randomized clinical trials. The publication years of the studies ranged from 2009 to 2020. Based on existing studies, interventions used to reduce SP following LS according to the surgical phases were included in this study.
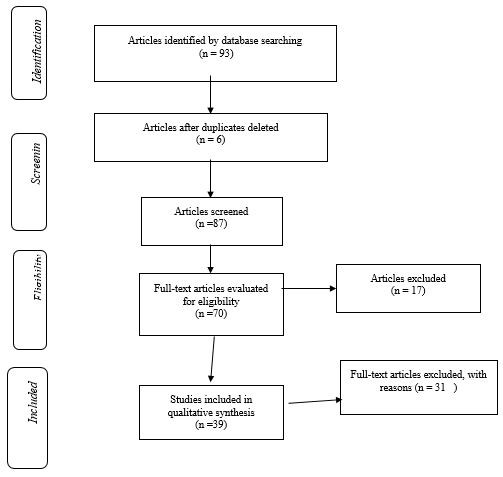
Figure 1. PRISMA diagram
Preoperative interventions
The presurgical phase is the period between decision making for operation and the start of the operation practice. In the present study, presurgical interventions involve many components and procedures performed before the patient’s admission to the hospital for a surgical operation to reduce postoperative SP.
Preoperative premedication with a drug: this drug included prescript premedication gabapentin (600 mg, oral) 30 min before surgery (30) and pregabalin (150 mg: 2 capsules of 75 mg), gabapentin (600 mg: 2 capsules of 300 mg), two hours before the beginning of operation (14), clonidine (0.2 mg clonidine HCL, oral) 90 min prior to induction (31), parecoxib (40 mg dissolved in 5 mL of normal saline) by intravenous injection 30 min before the surgery (32),and acetazolamide (5 mg/kg, oral) 1 h before the surgery (33) to prevent postoperative SP after LS.
Intraoperative interventions
The duration of the intraoperative phase is from admitting the patients to the operating room for the surgery until they are relocated to the recovery room. In the present study, intraoperative interventions involve measures andprocedures performed in the operating room during anesthesia and surgery to reduce postoperative SP.
These interventions includeintraperitoneal fluid therapy, an alternative used to reduce SP in LS, such as receiving intraperitoneal lignocaine (10 mL, 2%) versus bupivacaine (10 mL, 0.5%) by diluting every one in 10 mL of normal saline in LC (13), intraperitoneal ropivacaine (20 ml of 0.5%) by spraying 10 mL of solution into the hepato diaphragmatic space, 5 mL into the space within the liver and kidney and 5 mL in the area of the gallbladder (11), intraperitoneal 10 mL of bupivacain 0.5%, (in the space right under the diaphragm in the trendelenburg position) and 0.5 mg/kg of acetazolamide IV (intravenously) (30), intraperitoneal 50 ml of sodium bicarbonate ((NaHCO3) sodium bicarbonate 7.5% in 1000 ml of normal saline at 37 °C) injected in the surgical bed, superior surface of the liver below the right hemi diaphragm (31), intraperitoneal ketorolac (IP group: 1 mL of intravenous saline + ketorolac 30 mg in 250 mL of normal saline , 2- IV group: IV ketorolac 30 mg in 1 mL+ intraperitoneal normal saline 250 mL ) under standardized anesthesia (32), intraperitoneal instillation of 100 mg hydrocortisone (in 250 mL of normal saline) or 100 mg of bupivacaine (in 250 mL of normal saline) prior to insufflation of CO2 into the peritoneum (33-34), intraperitoneal 20 mL of Bupivacaine 0.25% in the gallbladder bed , abdominal CO2 insufflation during the resection of gallbladder (35), instillition of 20 ml of 0.5% bupivacaine intraperitoneal method at the terminal of laparoscopic surgery (36), intraperitoneal instillation of 100 mg of hydrocortisone in 250 ml of normal saline prior to insufflation of CO2 into the peritoneum (37), intraperitoneal ropivacaine 0.2% (1 ml/kg) instilled into the peritoneum before surgery (38), intrathecal fentanyl (23), and sedative effect of EMLA and trigger point injection (39) for postoperative pain management and relief after LS.
Another intraoperative intervention was drainage, which included forced evacuation of residual CO2 with the instillation of warm saline (1000-1500 mL) in the abdomen from one of the two supra inguinal ports until it spilled out of the staying open trocars (1), peritoneal gas drain (40), low-pressure CO2 through the pneumoperitoneum using paCO2 of 7–10 and 12–14 mm Hg (41), humidification of carbon dioxide by the HumiGard MR 860 Surgical Humidification System, CO2 insufflation with pressure set to 14 mm Hg, and an over gas flow limit of 6.5 L/min (42) for reducing postoperative SP in LS.
Low vs. Standard Pneumoperitoneum Pressure (43), spinal anesthesia with Subdiaphragmatic injections of 10 mL of 1% lidocaine at the port sites at the starting of the method (25), spinal/epidural block anesthesia (44), inactive-valve release on abdominal distension and active aspiration, (7), peritoneal suction with drainage, inserting Hemovac plastic passive drains in suprapubic position in a way that the opening of the drain was tangent to the peritoneum, without negative pressure for at least 24 h (45), peritoneal nebulization of Ropivacaine of ropivacaine 1% during LC (3). Bupivacaine 0.25% (50 mL solution), upivacaine ketamine with a whole volume of 50 mL intraperitoneal instilled solution combined with lung recruitment (46), a combined treatment of intraperitoneal normal saline and the PRM (47) use to reduce postsurgical SP in LS.
Neuromuscular blockade
Low insufflation pressures are plausible with neuromuscular blockade deeply throughout LS to mitigate SP after laparoscopy. In this method, continuous infusion of rocuronium was done after the induction, and titration was made based on the group appointment for maintaining either (PTC) the post-tetanic count at one to two in the deep NMB group, or train-of-4 (TOF) response at one-two in the moderate NMB group. Neuromuscular function was determined at the wrist by an acceleromyograph. This muscle relaxation power was retained by the completion of the abdominal fascia closure (48).
Pulmonary recruitment maneuver (PRM)
Five manual pulmonary inflations were offered at the completion of operation with an uppermost pressure of 60 cm H2O or 40 cm H2O (26, 49), which reduced residual pneunoperitoneum, thus reducing residual CO2 gas resulting in declined irritation of the phrenic nerve and reduction in post-laparoscopic SP.
Postoperative interventions
The extent of postoperative phase is from admitting the patients to the recovery room or PACU (post anesthesia care unit) and the time they are returned back to the operating unit until discharge. In the present study, postoperative interventions involve different measures and procedures after surgery to reduce postoperative SP.
This intervention consists of patients with suction drain positioned in the subhepatic space (28), exaggerated lithotomy position on SP after LC (5), and acupuncture treatments registered according to STRICTA guidelines (50) for pain relief after LS.
The findings of the meta-analysis were reported 6, 12, and 24 hours after LC. The results showed that there was heterogeneity between studies (I2 = 69.7%, P = 0.037, Q = 6.60) 6 hours after the operation in the intervention group.
Therefore, the studies and final results of the effects of the studies were combined using the SMD and random method. The results of the intraoperative intervention show that the mean VAS score is 1.46 with CI 95%: (-0.32, 3.24) in intervention group 6 hours after surgery.
There were homogeneity among studies (I2 = 1.5%, P = 0.362, Q = 2.03) in the control group 6 hours after operation. Therefore, studies and final results of the studies were combined using a fixed effects model. The estimated mean VAS score in the control group is 1.87 with CI 95%: (0.79, 2.94) at 6 hours post-operation. The VAS score of control group is greater than that of intervention group at 6 h after surgery (Figures 2 and 3).
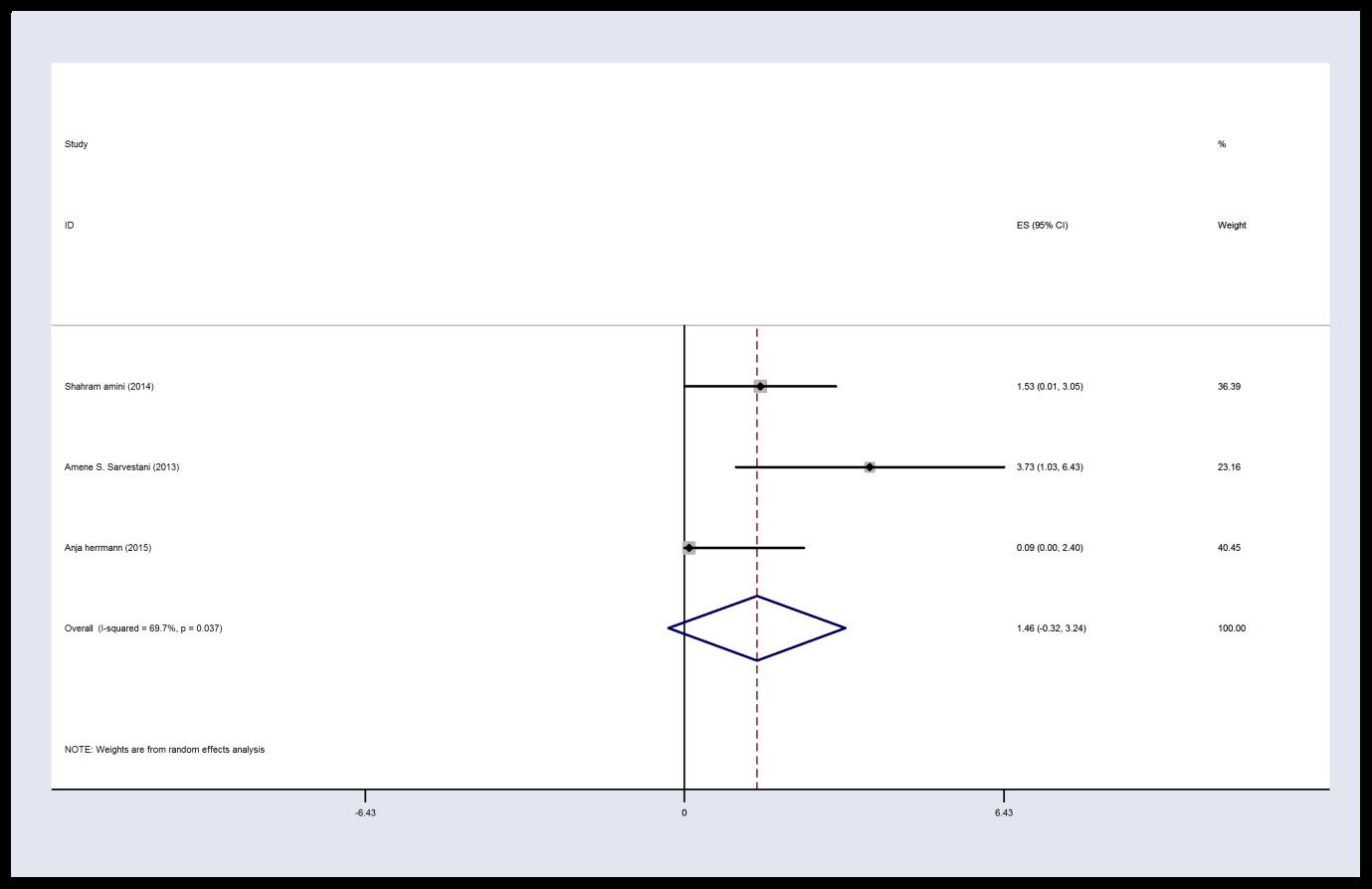
Figure 2. Forest plot of Visual Analog Scale score in the intervention group 6 h post-operation
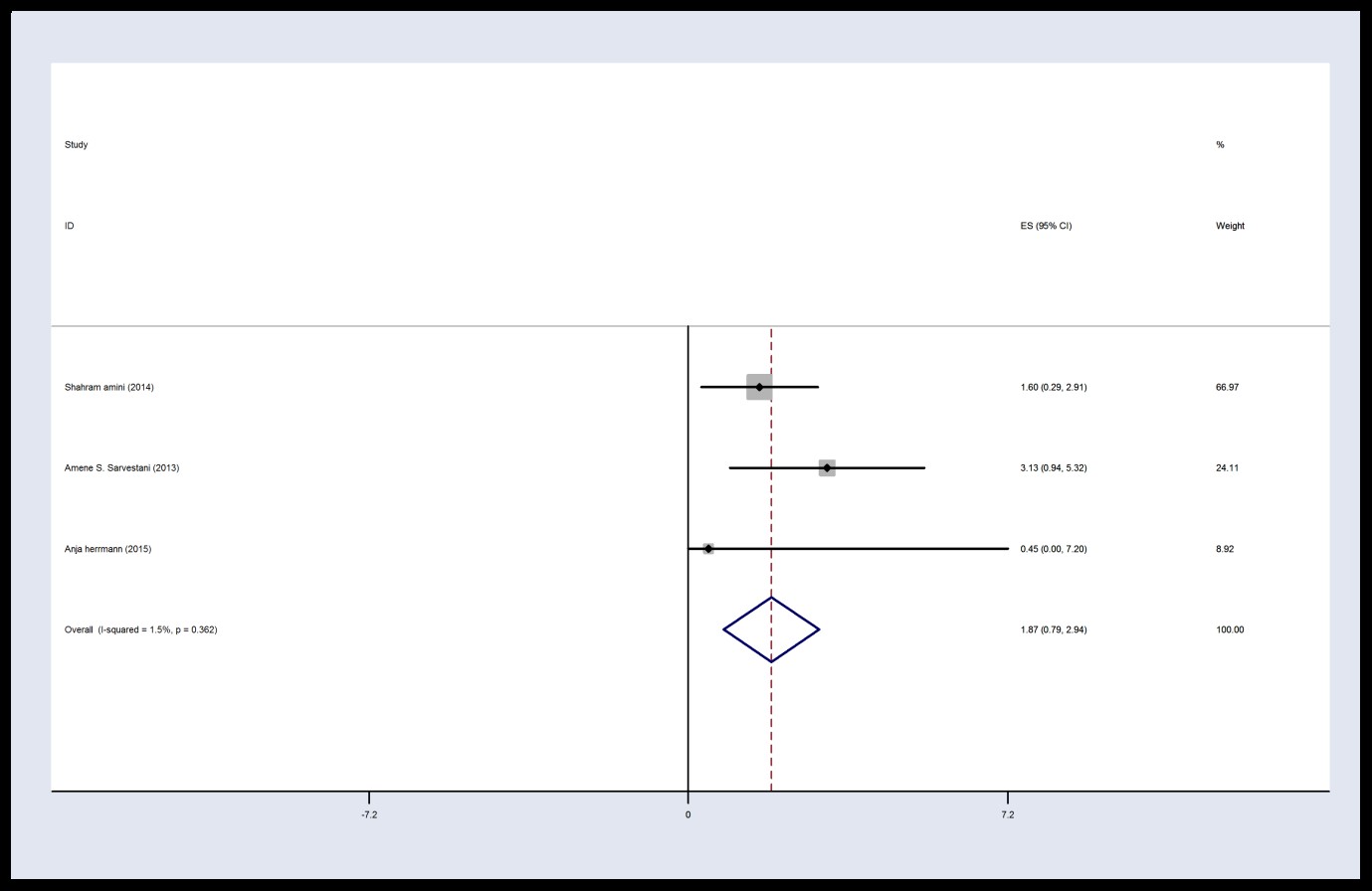
Figure 3. Forest plot of Visual Analogs Scale score in the control group 6 h post-operation
There was homogeneity between studies based on the finding of this meta-analysis 12 h post-operation in the intervention group (I2 = 0, P = 0.57, Q = 1.12). Thus, the results were combined according to the type of data obtained from the fixed effects model. These results demonstrated that the average of VAS score in intervention group was 2.06 with CI 95%: (0.91, 3.20) 12 h post-operation.
There was no homogeneity between studies in the control group at 12 hours post-operation (I2 = 62%, P = 0.072, Q = 5.27). Hence, the results were combined according to the type of data obtained from the random effects model. The results demonstrated that mean VAS score in the control group was 2.35 with CI 95%: (0.571, 4.13). The VAS score of the intervention group was lower than that of the control group 12 hours following surgery (Figures 4 and 5).
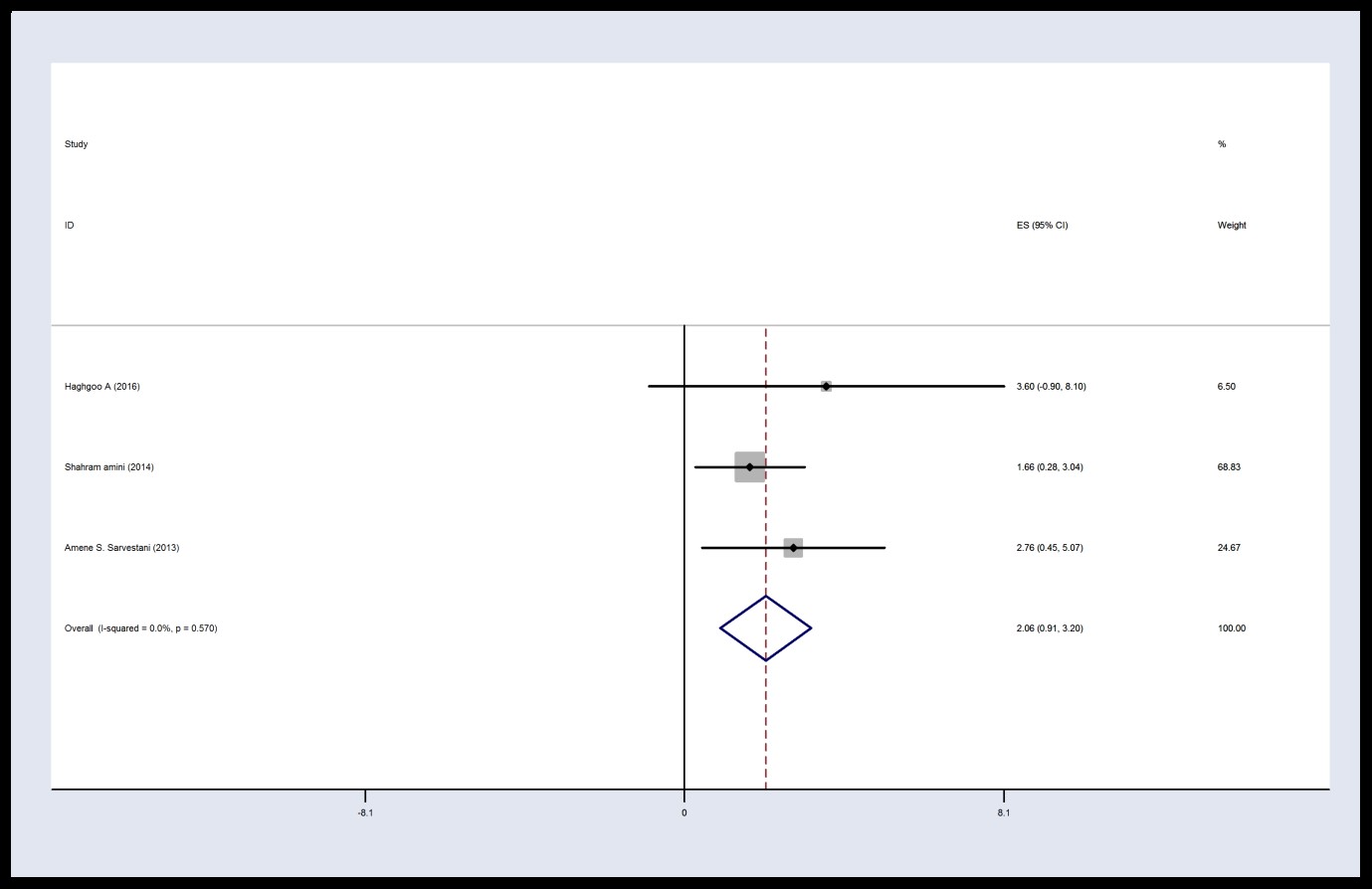
Figure 4. Forest plot of Visual Analog Scale score in the intervention group 12 h post-surgery
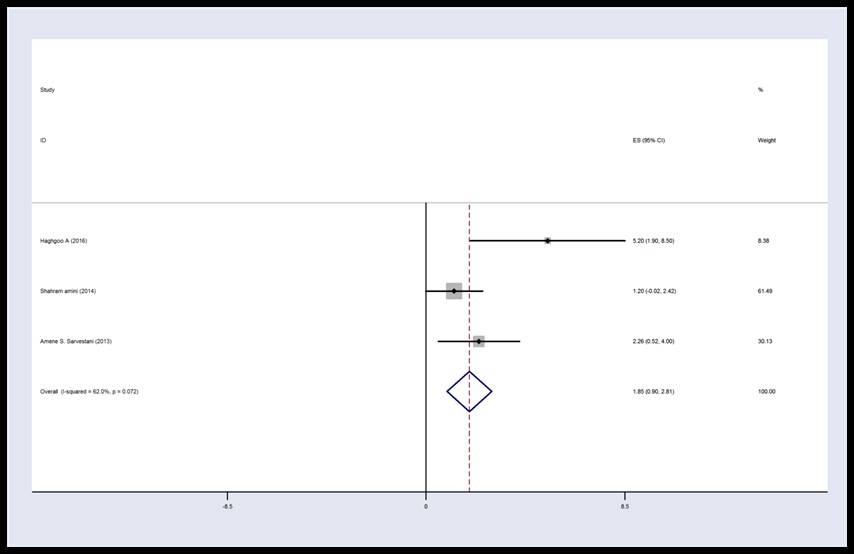
Figure 5. Forest plot of Visual Analog Scale score in the control group 12 h post-operation
Based on the finding of meta-analysis in the intervention group (I2 = 0, P = 0.83, Q = 0.88) 24 hours post-operation, there was homogeneity between studies. Therefore, the results were combined according to the type of data obtained from the fixed effects model. The results of intraoperative intervention show that the average VAS score in the intervention group is 0.96 with CI 95%: (-0.21, 2.13) 24 hours post-operation.
In the control group within twenty four hours after operation (I2=20%, P = 0.29, Q = 3.75), there was homogeneity between studies. Hence, the results were combined according to the type of data obtained from the fixed mod el. The average VAS score twenty four hours post-operation in the control group is 1.27 with CI 95%: (0.33, 2.21), which is higher than that of the intervention group at 24 hours after operation. Table 1 indicates articles included in present systematic review in detail.
Table 1. studies included in this systematic review
| No | Title/Author/year/country/reference | Type of study | Sample size | Type of Intervention | Intervention group | Measurement tool | Result |
| 1 | Prevention of Postlaparoscopic Shoulder Pain by Forced Evacuation of Residual CO2/Suginami R and et al./2009/ Japan (1) | RCT(two groups) | 40 patient candidates for gynecologic laparoscopic surgery |
Intraoperative interventions | installation of warm NS into the abdomen from one of the two suprainguinal ports until it spilled out of the remaining open trocars | VAS (visual analog scale) | VAS scores: in Group one enhancement on day one AM, reached a peak on day one PM, and declined gradually . in Group two stayed low overall during the investigation time |
| 2 | Preoperative Gabapentin to Prevent Postoperative Shoulder Pain After Laparoscopic Ovarian Cystectomy: A Randomized Clinical Trial/ Valadan M et al./ 2015/Iran (51) | double blind Clinical Trial | 40 female who were candidates to have elective laparoscopic ovarian cystectomy | Preoperative interventions | Case group received gabapentin 600 mg thirty minutes before operation as premedication |
VAS | PLSP ( Post Laparoscopic Shoulder Pain) was less in the gabapentin group paralleled with the placebo group.In gabapentin group the VAS scores were lower two, six, and twelve h, post surgery. |
| 3 | Peritrocal and Intraperitoneal Ropivacaine for Laparoscopic Cholecystectomy: A Prospective, Randomized, Double-Blind Controlled Trial/ Cha S.M et al./ 2012/ Korea (6) | prospective, randomized, double blind, controlled study |
80 patients assigned to four Groups A,B,C,D | Intraoperative interventions | Group A: received peritrocal and intraperitoneal saline. Group B: received peritrocal saline and intraperitoneal ropivacaine. Group C: received peritrocal ropivacaine and intraperitoneal saline.Group D: received peritrocal and intraperitoneal ropivacaine.These procedures were performed 10 min before the onset of operation. | VAS and PCA (patient controlled analgesia) | in Group B SP (shoulder pain) lower VAS scores from 4 to 48 h and in Group D from 2 to 12 h. |
| 4 | Clinical effects of intrathecal fentanyl on shoulder tip pain in laparoscopic total extraperitoneal inguinal hernia repair under spinal anaesthesia: A double-blind,prospective, randomized controlled trial/Sung T-Y et al./ 2013/ Korea (23) |
Double b linded,prospective,randomized |
36 patients in each group | Intraoperative interventions | Spinal anaesthesia was induced by intrathecal management with 2.6 ml of 0.5% hyperbaricbupivacaine (13 mg) and 10 mg fentanyl (0.2 ml) in the experimental group | Shoulder tip pain utilized grades of pain (0, 1,2,3); | inexperimental group had remarkably lessIntraoperative shoulder tip pain (9 [25%] versus 18 [50%]). |
| 5 | Drain after elective laparoscopic cholecystectomy.A randomized multicentre controlled trial/ Picchio. M/ 2012/ Italy (28) | randomized multicentre controlled trial | 53patients in each group | Postoperative interventions | patients have a suction drain positioned in the sub hepatic space |
VAS |
Not significant in either group |
| 6 | Intraperitoneal lignocaine (lidocaine) versus bupivacaine after laparoscopic cholecystectomy:Results of a randomized controlled trial/Rizwan Khan M/ 2012/ Pakistan (13) | Randomized controlled trial | 206 patients | Intraoperative interventions | Group L: 10 mL 2% lidocaine (lignocaine), Group B: 10 mL 0.5% bupivacaine, each diluted in 10 mL NS. All patients underwent standard perioperative anesthesia and analgesia protocol. | VAS | not significant at 5 time intervals |
| 7 | The Effect of Intraperitoneal Ropivacaine for Post-Operative Pain Management in Patients Undergoing Laparoscopic Cholecystectomy: A Prospective Double-Blind Randomized Control Study/ Singh D et al./ 2013/ India (11) | Prospective Double-Blind Randomized Control Study | 50patients: Group A:25 Group B:25 |
Intraoperative interventions | Group B: 20 ml of Ropivacaine0.5% ( 5 mL in the area of the gallbladder, sprayed 10mL of solution into the hepato-diaphragmatic space, and 5 mL into the space between kidney and liver ) | VAS | Pain in patients with VAS > 40, controlled with bolus of Diclofenac aqueous. in Group A SP existence with the highest |
| 8 | The Effect of Exaggerated Lithotomy Position on Shoulder Pain after Laparoscopic Cholecystectomy/ Aydemir O/ 2018/ Turkey (5) |
Semi experimental research | 102 patients | Postoperative interventions | lithotomy position was uded in the case group, as soon as SP began |
VAS and SPO2 |
Lithotomy position lowered the SP of the patients in case group. It incremented peripheral saturation levels of the patients more quickly paralleled with patients in control group receiving analgesics. |
| 9 | Treating Post laparoscopic Surgery Shoulder Pain with Acupuncture/ Kreindler G/ 2014/ Palestine (50) |
Randomized controlled trials | 25 patients |
Postoperative interventions | Acupuncture treatments done according to STRICTA guidelines. | VAS | A reduction in PLSP and general pain |
| 10 | Analgesic effect of trigger point injection and EMLA for shoulder pain in patients undergoing total laparoscopic hysterectomy: A randomized controlled study /Kim J E/ 2019/ Korea (39) | RCT (single-blinded) | 75 patients | Intraoperative interventions | TPI group:2mL ropivacaine 0.2% injections each shoulder, on 3points.. EMLA group: occlusive dressing with 2g EMLA utilizing semipermeable polyurethane membrane (Tegader) | VAS | The occurrence of SP declined in EMLA group paralleled to TPI groups .; the intensity of SP was reduce d in EMLA and TPI groups paralleled to control group. |
| 11 | Comparison of intraperitoneal bupivacain, acetazolamide,and placebo on pain relief after laparoscopic cholecystectomy surgery: A clinical trial/ Rahimzadeh P /2018/ Iran (30) | Randomized clinical trial | 60 patients | Intraoperative interventions | - group one: in trendelenburg position after removal of the gallbladder, 10 mL bupivacaine 0.5% was injected into the port location in space right below the diaphragm.Group two: gained 0.5 mg /kg acetazolamide IV. group three: as placebo saline IV. |
VAS | The highest outbreak of SP related to the placebo group and the lowest to acetazolamide |
| 12 | The effect of peritoneal gas drain on postoperative pain in benign gynecologic laparoscopic surgery: a double-blinded randomized controlled trial/Tharanon C/ 2016/ Thailand (40) | double-blinded randomized controlled trial | 45 females |
Intraoperative interventions | Intervention group a nasogastric tube No 14 was inserted 10 cm into the peritoneal cavity prior to closure of the patient’s abdominal wall. | VAS | The three highest mean differences in patients with longer operating time equal to 2 h were in total pain, |
| 13 | Maintaining Optimal Surgical Conditions with Low Insufflation Pressures is Possible with Deep Neuromuscular Blockade During Laparoscopic Colorectal Surgery/ Kim M.H/ 2016/ Korea (48) |
prospective ( parallel-group trial) | 72 patients | Intraoperative interventions | An online randomization generator was used to achieve moderate or deep neuromuscular block with continuous injection of rocuronium. | NRS (Numeric Rating Scale) |
Occurrence of postoperative SP was remarkably lower in deep group than in the moderate NMB group |
| 14 | Effects of Gabapentinoids Premedication on Shoulder Pain and Rehabilitation Quality after Laparoscopic Cholecystectomy:Pregabalin versus Gabapentin/ Nakhli M.S/ 2018/ Tunisia (14) | randomized, double-blind clinical trial |
90patients were included and randomized into 3 groups of 30 each (gabapentin, pregabalin, and placebo) |
Preoperative interventions | Group one: 150 mg of pregabalin. Group two: 600 mg of gabapentin. group three: capsules of placebo two h before the operation beginning |
VAS Spiegel Scale: for assessment of PONV incidence and sleeping quality |
Promedication with gabapentinoids can increase the quality of LS recovery by reducing SP, decreasing the incidence of PONV, and enhancing sleep quality |
| 15 | Comparing Hemodynamic Symptoms and the Level of Abdominal Pain in High- Versus Low-Pressure Carbon Dioxide in Patients Undergoing Laparoscopic Cholecystectomy/Mohammadzade A. R./2018/ Iran (41) |
double-blind randomized clinical trial | 60 patients | Intraoperative interventions | in groups 1 and 2: pneumoperitoneum with PaCO2 ( 7–10 , 12–14 mmHg ) |
VRS (Verbal Rating Scale) | in low-pressure group, the frequencies of SP were lower at the first hour after operation and at 4, 8, 12, and 24 hours post-surgery |
| 16 | Randomized controlled trial comparing the effects of usual gas release, active aspiration, and passive-valve release on abdominal distension in patients who have undergone laparoscopic cholecystectomy/ Tuvayanon W/2017/ Thailand (7) | Randomized controlled trial | 142 patients |
Intraoperative interventions | Group 1 or control group: gas release from the surgical wound without port release. Group 2: active gas aspiration via a subdiaphragmatic port. Group 3: passive-valve release via a subdiaphragmatic port valve opening |
no pain instrument | SP at 4 and 24 h compared with the control group in both intervention groups had decreased. In the active aspiration group, ambulation times in postoperative were shorter than the control group and passive-valve release group |
| 17 | Peritoneal Nebulization of Ropivacaine during Laparoscopic Cholecystectomy: Dose Finding and Pharmacokinetic Study/ Allegri M./2017/ Italy (3) | multicenter double-blind randomized controlled trial | 159patients |
Intraoperative interventions | Patients were randomized to receive peritoneal nebulization of 5mL (50mg group), 10mL (100mg group), or 15mL (150mg group) of ropivacaine1%. |
NRS | Nebulization of 50mg of ropivacaine had the same effect of 100 or 150mg on SP, nausea and vomiting in postoperative, hospital discharge timing and activity resumption. |
| 18 | Effect of Oral Clonidine on Shoulder Tip Pain and Hemodynamic Response After Laparoscopic Cholecystectomy: A Randomized Double Blind Study/Mirhosseini H/ 2017/ Iran (52) |
randomized controlled clinical trial | 60 patients |
Preoperative interventions | clonidine group: 0.2mg Clonidine HCL TAB, Toliddaru, Iran oral and() placebo group: vitaminC tablets ninety minutes prior to induction |
VAS | Considerable difference in severity of PLSP between two groups at reverse from anesthesia, 4 and 8 h after the surgery |
| 19 | The impact of a pulmonary recruitment maneuver to reduce post-laparoscopic shoulder pain: A randomized controlled trial/ Ryua K./2017/ Korea (49) |
randomized controlled trial | 90 Patients undergoing gynecologic laparoscopy | Intraoperative interventions | PRM content: five manual pulmonary inflations was used at the end of operation with a maximum pressure of 40 or 60 cmH2O | VAS | The PLSP scores in intervention groups were notably lower at 24 and 48 h postoperatively than in control group. |
| 20 | Parecoxib increases muscle pain threshold and relieves shoulder pain after gynecologic laparoscopy:a randomized controlled trial/ Zhang H./2016/ China (53) | randomized controlled trial(double-blind, and placebocontrolled Study) |
120 patients |
Preoperative interventions | parecoxib Group: 40 mg dissolved in 5 mL NS was IV infused 30 minutes before the operation and 8 and 20 h after the operation. |
VAS, PPT evaluate pressure algometer at bilateral shoulder muscles |
PPT levels of shoulder muscles in preoperative correlated with the intensity of SP after surgery. PPT levels of shoulder muscles, but not of forearm muscles, declined after operation. |
| 21 | Pulmonary Recruitment Maneuver for Reducing Postoperative Shoulder Pain Incidence After Laparoscopic Gynecologic Surgery: A Prospective Randomized Controlled Trial/ Sutchritpongsa P/ 2015/ Thailand (26) |
Prospective Randomized Controlled Trial | 160 women patient |
Intraoperative interventions | Manual pulmonary inflation accomplished by the anesthetist with a positive pressure of 40 cmH2O for five times and the last positive pressure was held for five seconds. |
NRS | SP occurrence in intervention and control groups were 6.8% and 6.8%, 33.3% and 24.3% , 41.3% and 33.8% after the surgery,respectively 12, 24h postoperatively. |
| 22 | Postoperative pain relief after laparoscopic cholecystectomy: intraperitoneal sodium bicarbonate versus normal saline/ Saadati K./2016/ Iran (31) |
double blind randomized clinical trial | 150 patients | Intraoperative interventions | Group A (NS group):1000 ml NA 0.9%. Group B (sodium bicarbonate group):50 ml sodium bicarbonate 7.5%in 1000 ml NS at a temperature of37C infused in the operation bed, under the right hemi diaphragm and superior surface of the liver. | VAS | Left SP was remarkably lower only between non-washing group and sodium bicarbonate group 6, 18, and 24 h postoperatively. no difference between the 3 intervention groups in right SP, port site incisional pain and back pain. |
| 23 | The Use of Peritoneal Suction Drainage to Reduce Shoulder Pain Caused by Gynecological Laparoscopy/ Haghgoo A/ 2016/ Iran (16) | randomized clinical trial | 92 patients | Intraoperative interventions | Closed with drainage, Hemovac plastic passive drains inserted in suprapubic position in a way that the opening of the drain was tangent to the peritoneum,without negative pressure for at least 24h. | VAS | After surgery (12 and 24 h), SP was lower in the group with drainage at 48 h post operation; there was no difference between mean VAS scores for all groups |
| 24 | Insufflation with Humidified and Heated Carbon Dioxide in Short-Term Laparoscopy: A Double-Blinded Randomized Controlled Trial/ Herrmann A/ 2015/ Germany (42) | A Double-Blinded Randomized Controlled Trial |
97 patient treatment | Intraoperative interventions | pneumoperitoneum was maintained with cold, dry CO2 or with warm, humidified CO2 | VAS | SP 6 h post surgery decreased in the case group. |
| 25 | Low vs Standard Pneumoperitoneum Pressure During Laparoscopic Hysterectomy: Prospective Randomized Trial/Bogani G/2014/ Italy (43) |
Randomized controlled trial | 44Women were | Intraoperative interventions | Undergoing MLH with utilization of either LPP. | VAS |
Occurrence and severity of SP at 1 and 3 h post surgery was lower in LPP group than in the SPP group, but 24 hours between-group no differences were detected |
| 26 | Intraperitoneal ketorolac for post-cholecystectomy pain: a double-blind randomized-controlled trial post-cholecystectomy/ Murdoch J /2016/ Canada (32) |
a double-blind randomized-controlled trial | 120 patients undergoing elective laparoscopic cholecystectomy |
Intraoperative interventions | 1-IP group:ketorolac 30 mg in 250 mL NS 1 mL + IV saline. 2- IV group: intraperitoneal NS 250 mL + IV ketorolac 30 mg in 1 mL under standardized anesthesia | VAS | SP and resting pain were decreased with IV and IP ketorolac paralleled with Control, but there was no difference between IV and IP groups. |
| 27 | Premedication With Single Dose of Acetazolamide for the Control of Referral Shoulder Pain After Laparoscopic Cholecystectomy/Movassaghi R/2015/Iran (54) |
randomized-controlled clinical trial study Divided into two groups randomly (intervention and control). |
70 subjects | Preoperative interventions | case group: 5 mg/kg acetazolamide oral 1 hour |
VAS | There was not significant difference in SP between groups 2, 4, 6, 8, 10, 12, and 24 h after LS. |
| 28 | Comparing the Impact of Intraperitoneal Hydrocortisone With Bupivacaine on Postoperative Pain/ Amini S/ 2014/Iran (33) |
RCT (double blind) |
63 patient | Intraoperative interventions | Intraperitoneal instillation of 100 mg hydrocortisone in 250 mL NS or 100 mg bupivacaine in 250 mL NS before insufflation of CO2 into the peritoneum for post surgical pain relief | VAS | In the hydrocortisone group no significant SP and abdominal pain scores were observed parallel to the bupivacaine group. |
| 29 | Intraperitoneal Bupivacaine Effect on Postoperative Nausea and Vomiting Following Laparoscopic Cholecystectomy/Yari M/ 2014/ Iran (34) | a double-blind randomized clinical trial- |
conducted on 66 patients | Intraoperative interventions | Bupivacaine group: 20 mL bupivacaine 0.25% in the gallbladder bed after abdominal CO2 insufflation and after cut of gallbladder(before and after cholecystectomy) | VAS) at 1, 2, 3 | Both groups for opioid consumption, during 4 h post-surgery were similar. |
| 30 | Intraperitoneal Hydrocortisone plus Bupivacaine administration For Pain Relief after Laparoscopic Cholecystectomy, A Comparison with Bupivacaine Alone/ Sabzi A/ 2014/ Iran (35) | Double-blind, randomized clinical trial. | Sixty two patients | Intraoperative interventions | intraperitoneal instillation of 100 mg bupivacaine in 250 ml NS or 100 mg hydrocortisone plus 100 mg bupivacaine in 250 ml NS before insufflation of CO2 into the peritoneum | VAS | in the hydrocortisone plus bupivacaine group remarkably lower SP and abdominal pain scores were observed. |
| 31 | Intraperitoneal hydrocortisone for pain relief after laparoscopic cholecystectomy/ Sarvestani A. S et al/ 2013/ Iran (37) |
RCT (double-blind) |
62 patients | Intraoperative interventions | Intraperitoneal instillation of 250 ml NS or 100 mg hydrocortisone in 250 ml NS before insufflation of CO2 into the peritoneum. | VAS | In hydrocortisone group notably lower SP and abdominal pain scores were observed. |
| 32 | Role of intraperitoneal ropivacaine in laparoscopic appendicectomy: a prospective, double-blinded randomized control Australian study/ Huang YY/ 2019/ Australia (38) |
RCT (double-blinded placebo versus)control trial | 86 patients | Intraoperative interventions | 0.2% ropivacaine. One milliliter per kilogram was subsequently instilled into the peritoneum at the end of surgery | PCA | there was a significant decrease in the number of times the PCA button was compressed in the ropivacaine group paralleled to the NS group |
| 33 | Comparative evaluation of intraperitoneal bupivacaine and bupivacaine ketamine combined with lung recruitment for reducing postoperative shoulder pain in laparoscopic cholecystectomy/ Mostafa R.H/ 2018/ Egypt (46) | randomized, double-blinded study |
40 patients of either sex | Intraoperative interventions | Bupivacaine group: 50 mL solution of bupivacaine 0.25% intraperitoneal instilled solution. Bupivacaine Ketamine Group: 0.5 mg/kg ketamine mixed with bupivacaine 0.25% with a total volume of 50 mL intraperitoneal instilled solution. | VASand hemodynamics assess | ketamine bupivacaine admixture had made dramatic decrease in SP |
| 34 | Spinal Anesthesia and Spinal Anesthesia with Subdiaphragmatic Lidocaine in Shoulder Pain Reduction for Gynecological Laparoscopic Surgery: A Randomized Clinical Trial/ Asghari Z/2017/ Iran (25) | clinical trial | 84 patients | Intraoperative interventions | Groups A: Spinal Anesthesia Groups B:Spinal Anesthesia with Subdiaphragmatic injections of 10 mL of lidocaine 1% at the port sites at the beginning of the procedure Group C: General Anesthesia |
VAS | The intensity of pain in patients at all-time intervals after operation was parallel between groups |
| 35 | Spinal/epidural block as an alternative to general anesthesia for laparoscopic appendectomy: a prospective randomized clinical study/ Erdem VM et al./2018/ Turkey (44) | a prospective randomized clinical study | Fifty patients |
Intraoperative interventions | SEA group: needle technique for combined spinal/epidural anesthesia. Local anesthesia: 2 ml of lidocaine 2% epidural anesthesia: 10 ml of bupivacaine 0.5%, 25 μg of fentanyl and 5 ml of isotonic saline. |
VAS | Postsurgical right SP was remarkably higher in the GA group paralleled to the SEA group. |
| 36 | Post Laparoscopic Reduction of pain By combining intraperitoneal normal saline and the pulmonary Recruitment maneuver (POLAR BEAR trial). RCT to estimate reduction in pain after laparoscopic surgery when using a combination therapy of intraperitoneal normal saline and the pulmonary recruitment maneuver/ van Dijk J et al./2017/ Dutch (47) | RCT | 127 patients | Intraoperative interventions | The upper abdomen is filled with NS infusion in Trendelenburg position. Then a pulmonary recruitment maneuver with a pressure of 40 cm H2O. The trocar sleeve valves will be left open, so CO2 can escape the abdominal cavity. | VAS | may decrease post-laparoscopic pain in women with LS |
| 37 | To study the analgesic effect of instillation of 20 ml 0.5% bupivacaine intraperitoneal route at the end of laparoscopic surgery with control 20 ml 0.9% saline intraperitoneally/ Inugala RR, et al./2016 (36) | Randomized Controlled Trials |
80 patients | Intraoperative interventions | Group Bupivacaine: 20 mL Bupivacaine 0.5% intraperitoneal instillation; postoperative period | VAS | Bupivacaine group had a better postosurgical pain relief in the first 6 hours with not complications. |
| 38 | Comparative study between levobupivacaine with clonidine and ropivacaine with clonidine in thoracic epidural block for laparoscopic cholecystectomy. Journal of Evolution of Medical and Dental Sciences. Pandey A, et al./2015/ India (55) | randomized single-blind controlled trial |
60 patients | Intraoperative interventions | Group I: epidural anesthesia 0.5% levobupivacaine with 1.2 µg/kg clonidine. Group II: epidural anesthesia 0.75% ropivacaine with 1.2 µg/kg clonidine |
---- | In both the groups, throughout the intervention, mean blood pressure levels and heart rate were significantly lower or parallel to the baseline levels. |
| 39 | Minilaparoscopic Versus Conventional Laparoscopic Hysterectomy:Results of a Randomized Trial. Ghezzi F, et al./2011/ Italy (56) | Randomized Trial | 76 patients | Intraoperative interventions | Group I: LH (Laparoscopic Hysterectomy) - Group II: MLH (Minilaparoscopic Hysterectomy | VAS | Not difference in pain scores at 1, 3, 8, and 24 h after operation between groups. |
Discussion
In this systematic review and meta-analysis evaluated interventions reduced SP following LS. The results of articles included in this systematic review showed that preoperative (e.g., gabapentin, clonidine, parecoxib, and acetazolamide) (14, 51-53), intraoperative (e.g., intraperitoneal ropivacaine, lignocaine, and ketorolac) (6, 11, 13, 30), and postoperative (e.g., suction drain placed in the subhepatic space and acupuncture treatments) (28, 50) interventions significantly mitigated SP after laparoscopy.
The reviewed investigations showed that the residue of CO2 gas following LC could persist in peritoneal space for a duration of 24 h post-operation. Therefore, a high percentage of patients would experience SP after LS due to CO2, which will significantly affect gastrointestinal symptoms, such as nausea and vomiting, postoperative activity, and patient satisfaction with LS (49).
Among the studies that were analyzed in this meta-analysis, those of Amini (2014) and Sarvestani (2013) examined the influence of intraperitoneal hydrocortisone alone with bupivacaine on postsurgical pain of LC (33،37).
Sarvestani (2013) provided standard anesthesia and randomly injected 250 ml of NS (normal saline) or 100 mg of hydrocortisone in 250 ml of NS (normal saline) in the peritoneum before injecting carbon dioxide into patients. Abdominal pain and SP scores were notably lower in hydrocortisone group in the PACU (recovery room) six, twelve, and 24 hours after operation. These results showed that intraperitoneally injected hydrocortisone reduced pain after (LC) with no considerable unwanted consequences (37). Amini (2014) in his study treated the patients with standard anesthesia and randomly injected themwith100 mg of hydrocortisone in 250 ml of NS or a hundred mg of bupivacaine in 250 ml of NS in peritoneum before CO2 injection. Patients were then evaluated for postoperative analgesia, nausea, and vomiting. The result showed that intraperitoneally administered hydrocortisone and bupivacaine before the penetration of CO2 gas could reduce postoperative pain and the need for analgesia. No postoperative side effects were observed in LC (33).
Several methods have been reported for the decrease of post-laparoscopic pain by intraperitoneal injection of hydrocortisone, bupivacaine and other drugs. These include received intraperitoneal lignocaine (10 mL 2%) versus bupivacaine (10 mL 0.5%) by diluting one in 10 mL of normal saline (13), intraperitoneal infusion of 100 mg hydrocortisone plus and 100 mg bupivacaine (in 250 ml of NS) or 20 mL bupivacaine 0.25% in gallbladder bed during abdominal CO2 insufflation and resection of gallbladder (34), intraperitoneal instillation of 100 mg bupivacaine (in 250 ml of NS) (35), instillation of 20 ml 0.5% bupivacaine intraperitoneal method (36), intraperitoneal ropivacaine 0.2% (1 ml/kg) instilled into the peritoneum (38).
Another study included in the meta-analysis was that of Haghgoo (2016) who used peritoneal suction drainage to reduce SP in gynecological laparoscopy. The researchersdivided the patients randomly into two groups, one with drainage and the other without drainage. In all patients, the abdominal cavity was washed with 1.5 L of warm saline at the completion of the operation. For patients whose abdomens were closed by drainage, the hemovac plastic inactive drains were placed in suprapubic position. The opening of drains was tangential to the peritoneum and without pressure. The drains were placed at least for 24 h (45). Drainage in the peritoneal cavity is believed to reduce postoperative pain by removing CO2 gas. Some other studies reported that the use of drainage reduced the overall pain after surgery. However, the study of Georgiou (2011) showed that patients with drainage and those without drainage were not significantly different after cholecystectomy (57).
PRM is one of the methods for the evacuation of CO2 gas from the abdominal cavity in LS. This mechanism reduces the intensity of SP and so reduces PCA (patient-controlled analgesia) an alternative to palliate SP after surgery (2).
A study by Ryu (2017) showed that PRM significantly reduced residual pneunoperitoneum, thus a reduction in residual CO2 gas resulted in declined botheration of the phrenic nerve and consequently reduced PLSP (49). Finding of a research by Khanna (2013) revealed that PRM caused pulmonary edema in pressure between forty and sixty cm of water, increased lung volume, and decreased diaphragm, resulting in the remaining discharge of CO2 from the abdominal cavity, thus reducing patients' postoperative SP (58). Sharami (2010) performed PRM with pressure 40 cm of water at terminal of LS and noticed a considerable reduction in intensity of SP in patients four, twelve, twenty four, and forty eight hours after operation (59).
According to finding of this meta-analysis, the use of PRM by Pergialiotis (2015) seemed to be a simply executed procedure with the potential prevention of post-laparoscopic SP (60).
Various pharmacological and mechanical methods have been utilized to palliate SP after LS. In an investigation included in the meta-analysis, Ghezzi (2011) compared SP in patients after laparoscopic hysterectomy (LH) versus mini laparoscopic hysterectomy (MLH). In this study, patients underwent LS with 5 mm ports (LH) or a needle approach using 3 mm tools (mini MLH). The size of the ports could be diminished with confidence without adversely affecting the surgeon's capability to accomplish LH. The mentioned research indicated that the reduced size of the ports led to no significant decrease in postsurgical pain and receiving analgesia in patients with LH (56). However, some articles, like Novitsky (2005), Huang (2003) and Lau (2005) reported that postoperative pain was less than LC in mini laparoscopy (61-63).
Another study included in meta-analysis was conducted by Herrmann (2015), who examined the effect of insufflation with humidified and heated carbon dioxide on pain after laparoscopic vaginal hysterectomy. All patients were administered standard anesthesia and then randomized for a case group of pneumoperitoneum with cold CO2 (room temperature), dry (0% humidity), and for an intervention group of warm CO2 (35 ± 2 °C), and wet (98% humidity) CO2. For CO2 infiltration, the pressure was set to 14 mm Hg and a high gas flow was limited to 6.5 L per minute. The finding of the research demonstrated that the penetration of hot and humid CO2 significantly reduced SP, particularly in the initial 6 h post-operation, as well as the need for morphine (42). However, studies by Yu (2013) and Sammour (2010) on effect of hot and humid CO2 gas on short-term intraoperative and postoperative consequences showed that postoperative pain scores have equivalent postoperative recovery parameters in both intervention and control groups (64, 65).
Limitations
The limitation of this study is the inclusion of publications only from ISI Web of Science, PubMed, MEDLINE, Cochrane, ProQuest, Scopus, Google Scholar, and Scientific Islamic Database (SID); databases from 2009-2020, and other articles in other databases were not included.
Conclusion
Our findings showed that patients experience SP after LC. This pain will also have a significant effect on the occurrence of gastrointestinal symptoms, such as nausea and vomiting, the patients’ postoperative activity and especially their satisfaction with LS. However, studies with the intervention of accelerated CO2 gas excretion from the abdominal cavity revealed that both SP after laparoscopy and the need for analgesia were significantly reduced in patients. In addition to relieving SP and reducing analgesia, these interventions reduced nausea in patients of the intervention group. Therefore, it can be concluded that the administration of pharmacological or and none pharmacological interventions to reduce SP in LS will accelerate postoperative activity, recovery, and satisfaction of patients, in addition to the relief of pain.
Acknowledgements
The authors are grateful to Sabzevar University of Medical Sciences, non-communicable disease center.
This article is derived from a research approved by Sabzevar University of Medical Sciences with the code 99001 and ethics code: IR.MEDSAB.REC.1399.031
Conflicts of Interest
We declare no conflict of interest regarding this manuscript.
Funding
None.
Received: 2022/11/11 | Accepted: 2023/03/26 | Published: 2023/09/27
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |






