BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://journal.zums.ac.ir/article-1-7052-en.html


 , Hossein Bonakchi2
, Hossein Bonakchi2 

 , Sajjad Rahimi Pordanjani3
, Sajjad Rahimi Pordanjani3 

 , Seyedeh Zahra Shahrokhi4
, Seyedeh Zahra Shahrokhi4 

 , Sina Mohagheghi5
, Sina Mohagheghi5 

 , Mehdi Allahbakhshian Farsani6
, Mehdi Allahbakhshian Farsani6 

 , Mohammad Hossein Mohammadi *7
, Mohammad Hossein Mohammadi *7 


2- Dept. of Biostatistics, Faculty of Paramedical Sciences, Shahid Beheshti University of Medical Sciences, Tehran, Iran
3- Dept. of Epidemiology and Biostatistics, Semnan University of Medical Sciences, Semnan, Iran
4- Dept. of Biochemistry, School of Medicine, AJA University of Medical Sciences, Tehran, Iran
5- Dept. of Clinical Biochemistry, Faculty of Medicine, Hamadan University of Medical Sciences, Hamadan, Iran
6- Dept. of Hematology and Blood Banking, School of Allied Medical Sciences, Shahid Beheshti University of Medical Sciences, Tehran, Iran
7- Dept. of Hematology and Blood Banking, School of Allied Medical Sciences, Shahid Beheshti University of Medical Sciences, Tehran, Iran ,
✅ The management of AHSCT will be more efficient by considering the age, CD34+ CPK, and TDIG. For enhanced engraftment, adjusting the G-CSF injection days for <4 days and total dose of G-CSF on <4000 micrograms are suggested.
High dose chemotherapy, along with the autologous hematopoietic stem cell transplantation (AHSCT), was applied for over 50 years as a beneficial treatment for a wide range of malignancies (1, 2). AHSCT is a conventional and standard therapy for the management of multiple myeloma (MM) and lymphoma (3). Due to their many benefits, mobilized peripheral blood hematopoietic stem cells are used as a preferred source of hematopoietic stem cells (HSCs) (4). Granulocyte-colony stimulating factor (G-CSF), the primary mobilizer agent, is a desirable medication for mobilization because it facilitates affordable and predictable apheresis scheduling (5). A sufficient number of CD34+ HSCs or CD34+ count per kilogram (CD34+ CPK) is a critical factor for engraftment, and successful transplantation; however, in 5–40% of patients, CD34+ cells don’t reach desired number (6). Despite the lack of correlation among age, gender, bone marrow involvement, and mobilization efficiency (7), stress, tissue injury, exercise, and sterile inflammation result in higher rates of CD34+ HSC mobilization in the peripheral blood (8). Several risk factors, such as senescence, being affected by non-Hodgkin's lymphoma (NHL), the history of radiation therapy, lenalidomide consumption, having a low number of CD34+ cells in pre-apheresis peripheral blood samples (PAPBS), diabetes, and smoking, are involved in mobilization failure (5). However, the prediction of mobilization would be difficult and sufficient CD34+ CPK may mobilize in the individuals with risk factors while it may fail in individuals with no risk factors (5). Overall, mobilization-related parameters, particularly engraftment, may affect the results of HSCT (6, 9). It was shown that the 3-year survival rate is lower in poor mobilizers (patients having CD34+ CPK <2×106) than in those with sufficient mobilizers (33% vs. 71%, respectively) (10). The enhancement of mobilization protocols, and the prediction of poor mobilizers can reduce the costs of additional health care. Hence, the effects of G-CSF mobilization-related factors, and other AHSCT and patient’s related factors on the quality and quantity of harvested grafts and engraftment after AHSCT are studied.
Participants
Between October 2019 and May 2020, patients who were AHSCT candidates at the Taleghani Hospital's Bone Marrow Transplantation Department in Tehran, Iran, were included in the study. The following diseases were present in the patients: NHL, Hodgkin's diseases (HD), and MM. The exclusion criteria had infectious diseases, bleeding, splenomegaly, and using myelo-suppressive agents. All patients had received a myeloablative conditioning regimen, including Melphalan/Velcade for the patients with MM in a 1-day course and CEAM (Lomustine, Etoposide, Cytarabine, and Melphalan) for the patients with lymphoma in a 4-day course. The study is approved by the ethics committee with code IR.SBMU.REC.1398.157.
Participant’s data collecting
Data on procedures, medication dosages, regimens, periods of engraftment, and other topics were gathered from information in medical records, test data, and other sources. The patients had all been receiving G-CSF daily up to leukapheresis. Recorded total dose of infused G-CSF (TDIG), and the number of days of G-CSF infusion (NDGI) were collected. CD34+ cells per microliter of PAPBS that were analyzed by flow cytometry, were collected. Leukapheresis was performed by Spectra Optia (Terumo BCT, Lakewood, CO). Pre-apheresis white blood cell (WBC) count, time, and duration of apheresis, as well as the volume of processed blood and collected cells were collected. Moreover, CD34+ CPK, the number of CD45dim, WBC, and mononucleated cells (MNCs) in the apheresis products were collected. In the hospital, evaluation of CD34+ CPK was conducted based on the protocols established by the International Society of Hematotherapy and Graft Engineering (ISHAGE) (11). Hospitalization days, the time interval between the last injection of G-CSF and apheresis, neutrophil, and platelet (PLT) engraftment days were collected from medical records. Engraftment day was defined as the first day of three consecutive days with neutrophil and PLT counts of 500 cells/µl and 20×103 cells/µl, respectively that were collected (12).
Statistical analysis
The recorded outcomes of AHSCT were time of neutrophil and PLT engraftments. The predictive value of CD34+ CPK was evaluated for neutrophil and PLT engraftments using receiver-operating characteristic (ROC) analysis. The cut-off value for CD34+ CPK was determined by ROC analysis. The univariate and multiple variate analyses of time to event data were conducted using Cox proportional hazards model. Using the score process plot and the Kolmogorov-type supremum test (the significance threshold was 0.05), the assumption of proportional hazards was tested. The backward technique was used in this model's multiple variate analysis to identify the traits with the greatest predictive values. The significance level was set at 5% to eliminate variables from the model. A logarithmic transformation was used for non-normal variables and variables with large scales. For the univariate analysis of risk factor effects on WBC, MNC, CD34+ CPK, and CD34+ cell counts in PAPBS, generalized additive model was used. The significance level for the univariate analysis of Cox and generalized additive regression models was set at 10%. For a more detailed assessment, the patients were divided into two groups based on TDIG, and the correlation of TDIG with CD34+ CPK, CD34+ cell count in PAPBS, WBC counts at apheresis day, and the day of total engraftment was analyzed. SAS (version 9.4; SAS Institute Inc., Cary, NC, USA) and R (version 3.5.2) were used to analysis the data.
In general, 50 candidates for AHSCT were included, and the clinical characteristics of patients are shown in Table 1. The median time of neutrophils, PLTs, and the total engraftment (the engraftment of neutrophil and PLT), was 11 days, with the following ranges: 7-20, 9-19, and 9-21 days, respectively. The median numbers of CD34+ CPK and MNCs in the apheresis product were 3.5×106/kg (1-15.4) and 6.2×108/kg (3.3-17.5), respectively.
Table 1. Characteristics of patients (N=50)
| Features | Mean±SD /Median (Rang) / Frequency (%) | |
| Age (year) | 42.40±14.61 | |
| Gender | ||
| Male | 25(50) | |
| Female | 25(50) | |
| Diagnosis | ||
| MM | 24(48) | |
| HD | 15(30) | |
| NHL | 11(22) | |
| BMI Before transplantation | 26.94±5.46 | |
| BMI after transplantation | 26.72±5.41 | |
| NDGI (day) | 6 (5-9) | |
| TDIG (µg) | 4002±1147.75 | |
| WBC count at day of start of mobilization (×103/µl) | 6.05(2.4-22.1) | |
| Last injection of G-CSF to apheresis (h) | 6.5±4.83 | |
| WBC count at apheresis day (×103/µl) | 39.42±14.59 | |
| Neutrophil count on apheresis day (×103/µl) | 34.82±14.28 | |
| Platelet count on apheresis day (×103/µl) | 171.64±53.35 | |
| Apheresis duration (min) | 386.81±50.28 | |
| ACD volume for apheresis (ml) | 1507(950-3000) | |
| Total processed blood in apheresis (l) | 18.43±3.76 | |
| Apheresis bag volume (ml) | 406.14±84.11 | |
| WBC count in apheresis product (×108/kg) | 11.38±4.39 | |
| MNC count in apheresis product (×108/kg) | 6.20(3.3-17.5) | |
| CD45+ in apheresis product (%) | 98.05(95-99.5) | |
| CD45+dim in apheresis product (%) | 1.20(0.2-9.2) | |
| CD34+ CPK (×106/kg) | 3.5(1-15.4) | |
| CD34+ in PAPBS (/µl) | 45.85±29.73 |
Predictive values of CD34+ CPK for neutrophil and PLT engraftment
ROC analysis and the assessment of the area under the curve (AUC) were carried out to evaluate the predictive values of CD34+ CPK for neutrophil and PLT engraftment. AUC was 76.9% (62-92%) with a cut-off value of 3.5×106 (Figure 1A) for PLT. Therefore, it seems CD34+ CPK could be used as a fair predictive factor for PLT engraftment. AUC was 64.4% (44-84%) with a cut-off value of 3.4×106 (Figure 1B) for neutrophils. So, it appears that CD34+ CPK is a poor predictive factor for neutrophil engraftment.
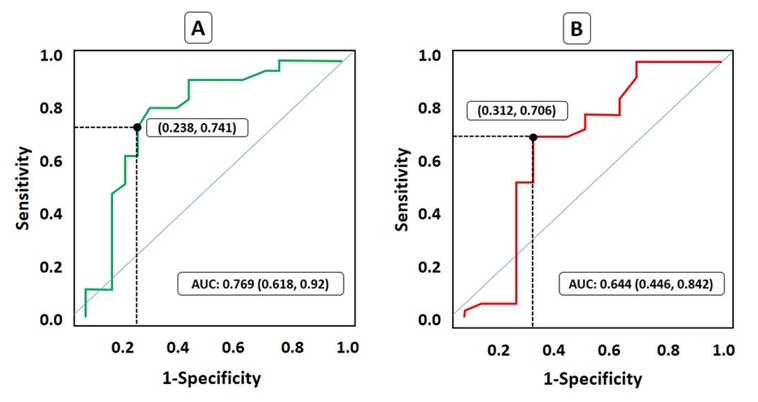
Figure 1. ROC assessment for the predictive value of CD34+ CPK for engraftment. Cut off points are shown.
A. Platelet engraftment, B. Neutrophil engraftment
Risk factors of PLT and neutrophil engraftment
PLT engraftment
The factors in Table 2 that are associated with PLT engraftment. Using the backward method to incorporate significant risk factors from the univariate model into the multivariate model, age and CD34+ CPK were found to be associated with PLT engraftment. Assuming the number of CD34+ CPK cells was fixed, a one-year increase in the age of patient’s results in an improvement of PLT engraftment by 5%. Assuming age does not affect the engraftment outcomes, the number of CD34+ CPK cells ≥3.5×106/kg led to the enhancement of PLT engraftment compared with those with CD34+ CPK <3.5×106. Regarding Figure 2, the patients with an age of over 42 years old and CD34+ CPK ≥3.5× 106 have better PLT engraftment outcomes than those with an age of ≤42 and CD34+ CPK <3.5× 106 (p-value: 0.02).
Table 2. The influence of risk factors on platelet engraftment
| Univariate | Multiple2 | |||
| Variables | HR (90% CI) | P-Value | AHR3 (95% CI) | P-Value |
| Age | 1.02(1.003-1.04) | 0.06* | 1.05(1.01-1.08) | 0.003** |
| Gender | 0.56 | |||
| Male | 1.25(0.66-2.36) | 0.56 | ||
| Female (RL1) | 1 | - | ||
| Diagnosis | 0.06* | NS |
||
| MM | 5.33(2.25-12.61) | 0.02 | ||
| HD | 3.35(1.35-8.34) | 0.12 | ||
| NHL (RL1) | 1 | - | ||
| BMI Before transplantation | 1.06(1.001-1.13) | 0.09* | NS | |
| BMI after transplantation | 1.06(1.004-1.13) | 0.08* | NS | |
| NDGI | 0.72(0.52-1.007) | 0.10 | NS | |
| TDIG4 | 0.11(0.03-.35) | 0.001* | NS | |
| WBC count in day of start of Mobilization | 1.06(0.99-1.13) | 0.11 | ||
| Last injection of G-CSF to apheresis | 0.97(0.89-1.05) | 0.54 | ||
| WBC count at apheresis day | 0.98(0.95-1.004) | 0.16 | ||
| Neutrophil count at apheresis day4 | 0.47(0.22 - 0.99) | 0.09* | NS | |
| Apheresis duration | 1.004(0.99-1.01) | 0.33 | ||
| ACD volume for apheresis4 | 1.89(0.31-11.51) | 0.56 | ||
| Total processed blood in apheresis4 | 4.97(0.90-27.47) | 0.12 | ||
| Apheresis bag volume | 1.001(0.99-1.005) | 0.52 | ||
| WBC count in apheresis product | 0.93(0.86-1.01) | 0.17 | ||
| MNC count in apheresis product | 0.92(0.82-1.04) | 0.28 | ||
| CD45+ % in apheresis product | 1.09(0.77-1.53) | 0.67 | ||
| CD45+dim % in apheresis product | 0.97(0.84-1.12) | 0.77 | ||
| CD34+ CPK (×106) | 0.002* | 0.0003** | ||
| =>3.5 | 3.78(1.82-7.85) | 0.002 | 6.27(2.29-17.41) | 0.0003 |
| <3.5(RL1) | 1 | - | 1 | - |
| CD34+ in PAPBS | 1.005(0.99-1.01) | 0.52 | ||
1: Reference Level, 2: Backward Selection, 3: Adjusted Hazard Ratio, 4: Logarithm scale, Underline: Borderline significant
*. Significant at 0.1
**. Significant at 0.05
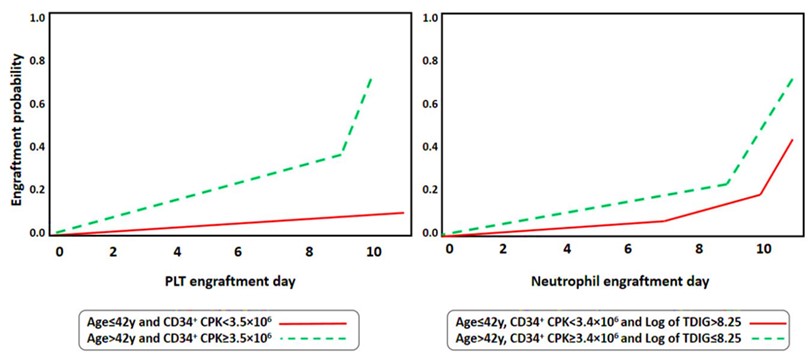
Figure 2. Cumolative incidence of PLT and Neutrophil engraftment based on Age, CD34+ CPK and TDIG
Neutrophil engraftment
Table 3 is showing risk factors for neutrophil engraftment. The significant risk factors in the univariate model were integrated with multiple-model using the backward method. The findings revealed that neutrophil engraftment was correlated with patient age, TDIG, and CD34+CPK. While the effect of other variables was fixed, a one-year increase in the age of patients improved the rate of neutrophil engraftment by 4%. Moreover, a one-unit increase in the logarithmic scale of TDIG declined the rate of neutrophil engraftment by 76%. Finally, CD34+ CPK ≥3.4×106/kg was associated with an improved time of neutrophil engraftment compared with the patients with CD34+ CPK <3.4×106. Regarding Figure 2, patients with an age >42, CD34+ CPK ≥3.4× 106, and TDIG ≤8.25 on the logarithmic scale have a higher rate of neutrophil engraftment compared with those with an age ≤42, CD34+ CPK <3.4× 106 and TDIG >8.25 (p-value: 0.032).
Table 3. The influence of risk factors on neutrophil engraftment
| Univariate | Multiple2 | |||
| Variables | HR (90% CI) | P-Value | AHR3 (95% CI) | P-Value |
| Age | 1.02(1.008-1.05) | 0.02* | 1.04(1.01-1.07) | 0.002** |
| Gender | 0.71 | |||
| Male | 1.13(0.64-1.99) | 0.71 | ||
| Female (RL1) | 1 | - | ||
| Diagnosis | 0.01* | NS |
||
| MM | 3.92(1.59-9.68) | 0.01 | - | |
| HD | 1.78(0.66-4.79) | 0.33 | - | |
| NHL (RL1) | 1 | - | ||
| BMI Before transplantation | 1.04(0.98-1.10) | 0.20 | ||
| BMI after transplantation | 1.05(0.99-1.11) | 0.16 | ||
| NDGI | 0.74(0.55-0.99) | 0.09* | - |
NS |
| TDIG4 | 0.20(0.06-0.58) | 0.01* | 0.24(0.06-0.89) | 0.03* |
| WBC count at day of start of Mobilization | 1.04(0.97-1.11) | 0.31 | ||
| Last injection of G-CSF to apheresis | 0.98(0.92-1.04) | 0.63 | ||
| WBC count at apheresis day | 0.97(0.95-0.99) | 0.08* | - |
NS |
| Neutrophil count at apheresis day4 | 0.47(0.23-0.95) | 0.07* | - |
NS |
| Apheresis duration | 1.002(0.99-1.008) | 0.59 | ||
| ACD volume for apheresis4 | 2.99(0.60-14.69) | 0.25 | ||
| Total processed blood in apheresis4 | 2.55(0.61-10.55) | 0.27 | ||
| Apheresis bag volume | 1 (0.99-1.003) | 0.94 | ||
| WBC count in apheresis product | 0.93(0.87-1.002) | 0.10 | - |
NS |
| MNC count in apheresis product | 0.86(0.77-0.97) | 0.05* | - |
NS |
| CD45+ % in apheresis product | 1.02(0.77-1.36) | 0.88 | ||
| CD45+dim % in apheresis product | 0.93(0.81-1.07) | 0.42 | ||
| CD34+ CPK (×106) | 0.01* | 0.03** | ||
| =>3.4 | 2.48(1.33-4.65) | 0.01 | 2.50(1.07-5.79) | 0.03 |
| <3.4(RL1) | 1 | - | 1 | - |
| CD34+ in PAPBS | 1.005(0.99-1.01) | 0.45 | ||
1: Reference Level, 2: Backward Selection, 3: Adjusted Hazard Ratio, 4: Logarithm scale, Underline: Borderline significant
*. Significant at 0.10
**. Significant at 0.05
Risk factors of mobilization
Table 4 lists the mobilization risk variables. The findings showed that NDGI and TDIG were the most prevalent and significant risk variables for CD34+ CPK and CD34+ cell count in PAPBS. The CD34+ CPK and CD34+ cell count in PAPBS decreased by 22% and 7.46%, respectively, in response to an increase in NDGI over one day.The results showed that the counts of CD34+ CPK and CD34+ cells in PAPBS were decreased (by 63% and 36.62%, respectively) in response to a one-unit increase in the logarithmic scale of TDIG.
Table 4. Univariate analysis for Risk factors of mobilization
| WBC count in apheresis product | MNC count in apheresis product2 | CD34+ CPK2 | CD34+ cell count in PAPBS | |||||
| Variables | Beta | P-Value | Beta | P-Value | Beta | P-Value | Beta | P-Value |
| Age | 0.03 | 0.42 | -0.001 | 0.64 | -0.003 | 0.60 | 0.45 | 0.21 |
| Weight before transplantation | -0.06 | 0.12 | -0.007 | 0.04* | 0.005 | 0.36 | 0.25 | 0.43 |
| NDGI | 0.41 | 0.46 | 0.03 | 0.47 | -0.25 | 0.001* | -7.46 | 0.09* |
| TDIG2 | 0.74 | 0.72 | -0.05 | 0.76 | -1.0003 | 0.0006* | -36.62 | 0.03* |
| WBC count at day of start of Mobilization | -0.06 | 0.68 | -0.004 | 0.76 | 0.02 | 0.27 | 0.74 | 0.50 |
| Last injection of G-CSF to apheresis | -0.05 | 0.70 | 0.004 | 0.71 | 0.01 | 0.56 | 1.45 | 0.23 |
| WBC count at apheresis day | 0.15 | 0.0001* | 0.009 | 0.008* | 0.002 | 0.69 | 0.41 | 0.18 |
| Neutrophil count at apheresis day2 | 5.43 | 0.0002* | 0.29 | 0.02* | 0.01 | 0.95 | 11.87 | 0.33 |
| Platelet count at apheresis day2 | 2.01 | 0.20 | 0.15 | 0.26 | 0.64 | 0.01* | 15.49 | 0.33 |
| Apheresis Time, Morning | 0.03 | 0.18 | 0.95 | |||||
| Yes | -3.46 | 0.03 | -0.18 | 0.18 | -0.01 | 0.95 | ||
| No (RL1) | - | - | - | - | - | - | ||
| Afternoon | 0.02 | 0.14 | 0.33 | |||||
| Yes | 3.34 | 0.02 | 0.17 | 0.14 | -0.21 | 0.33 | ||
| No (RL1) | - | - | - | - | - | - | ||
| Evening | 0.64 | 0.36 | 0.29 | |||||
| Yes | -0.57 | 0.64 | -0.09 | 0.36 | 0.19 | 0.29 | ||
| No (RL1) | - | - | - | - | - | - | ||
| Apheresis duration | 0.002 | 0.85 | 0.0004 | 0.96 | 0.001 | 0.56 | ||
| ACD volume for apheresis2 | -3.36 | 0.31 | -0.34 | 0.19 | 1.29 | 0.008* | ||
| Total processed blood in apheresis2 | -1.76 | 0.55 | -0.30 | 0.20 | 0.81 | 0.06* | ||
1: Reference Level, 2: Logarithm scale
*: Significant at 0.10
Correlation of TDIG with the mobilization and engraftment results in experimental groups
Patients were divided into two groups based on the mean values of TDIG on the logarithmic scale (8.25), and then the correlations were analyzed. In a group with TDIG values of >8.25, an increase in TDIG was negatively significant and correlated with the number of CD34+ CPK and CD34+ cells in PAPBS (Figure 3). On the other hand, in both experimental groups, a rise in TDIG was positively correlated with WBC levels on the apheresis day. Additionally, a rise in TDIG considerably slowed total engraftment in a group with TDIG > 8.25 (Figure 3).
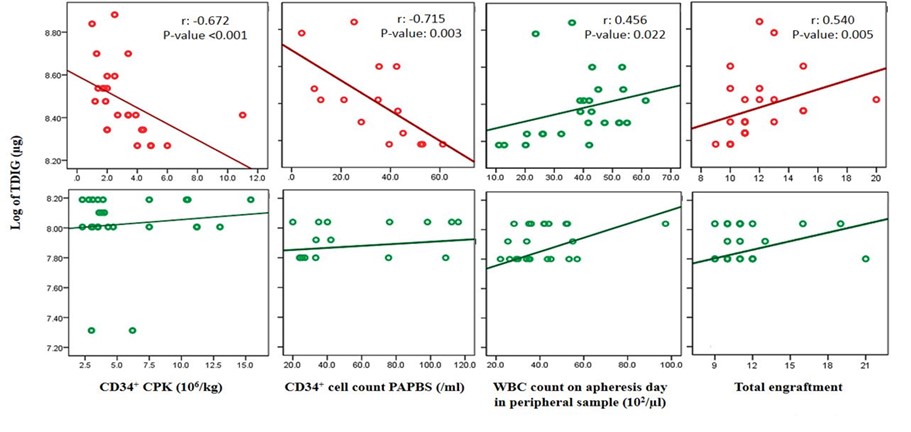
Figure 3. Correlation of TDIG in tow group (low and high) with CD34+ CPK, CD34+ cell count in PAPBS, WBC count at apheresis day and total engraftment
Discussion
Methods and strategies to increase mobilization efficiency are being evaluated to prevent mobilization failure, delayed recovery following transplantation, and additional costs (5). Insufficient mobilization may occur in the individuals without any risk factors (5, 13), and a total of 10-30% of AHSCT candidates are unable to mobilize a sufficient number of CD34+ cells (14). Therefore, a comprehensive assessment of all involved parameters seems to be necessary. Cellular engraftment, including neutrophils and platelets, affected by a group of factors, from chemotherapy (15) to transfusion-related iron overload mobilization (16), is considered the first and most significant outcome of AHSCT (17), and its precise prediction can be useful in the management and treatment process to prevent complications and high costs (18). The effect of medication administration on mobilization may be assessed by counting the cells in peripheral blood after mobilization and using apheresis products. The predictive value and impact of several parameters on the outcomes of apheresis and engraftment at our facility were examined in the current research.
Numerous studies showed that 3-5×106/kg of CD34+ CPK can largely guarantee rapid neutrophil, and platelet engraftment (5). The number of CD34+ CPK cells in the range of 1.5×106 to 2.5×106/kg cells may result in delayed platelet engraftment, and values above 1×106 may necessitate erythrocyte injection and even permanent engraftment failure (5). Based on to our results, CD34+ CPK is a poor predictor of neutrophil engraftment and a fair predictor of platelet engraftment. According to a study conducted by Demirer et al., CD34+ CPK >5×106/kg has no effect on improving the kinetics of neutrophil engraftment but could result in faster platelet recovery (19), while Weavwe et al. reported that CD34+ CPK >5×106/kg is a threshold to ensure rapid platelet and neutrophil engraftment (20). CD34+ CPK >10×106/kg is associated with faster neutrophil and platelet engraftment by 1-2 and 2-4 days, respectively (20, 21). Further research is needed to see if more CD34+ cells improve engraftment (5).
Our findings indicated that people over 42 years old, afflicted with MM, having CD34+ CPK >3.5×106/kg, are correlated with improved platelet engraftment. High neutrophil counts on the apheresis day and increased values of TDIG lead to delayed platelet engraftment. Other studies have shown that NDGI ≥3 days, CD34+ CPK <2×106/kg, low platelet counts (<150×103), and low hemoglobin concentrations in PAPBS lead to delayed platelet engraftment while having MM and being over the age of 28 results in enhanced platelet engraftment (22-24). Furthermore, based on the results of our study, age over 42 years, being afflicted with MM, and CD34+ CPK >3.4×106/kg lead to faster neutrophil engraftment, while the elevated number of MNCs in the apheresis product results in prolonged neutrophil engraftment. In contrast to our findings, Grubovic et al. (25), found that an increase in MNC counts in the apheresis product is associated with faster neutrophil engraftment, whereas one study found that an increase in MNC counts is not associated with the kinetics of neutrophil engraftment in the apheresis product (19). The quantity of WBCs (including MNCs) is suggested to rise in tandem with an extension of the mobilization time; however, as the cells mature, fewer immature HSCs may need to be injected into patients (26). Additionally, it has been shown that age and long-term mobilization have a negative connection with neutrophil engraftment (25).
Some factors are effective on apheresis products, as Gambell et al. reported a linear association among CD34+ cell counts in PAPBS and CD34+ CPK, so that as the number of CD34+ cells in PAPBS is increased by two folds, the count of CD34+ CPK is doubled (27). PAPBS contains more than 50/µl of CD34+ cells, results in CD34+ CPK > 2.5×106/kg (19). When the number of CD34+ in PAPBS is <20/µl, it is commonly considered poor mobilization, which may vary depending on different centers, patient types, treatment regimens, and mobilization protocols (28). It has been reported that the number of white blood cells (WBCs) and micronucleated cells (MNCs) in the pre-apheresis blood sample (PAPBS) can be used to predict the optimal time to perform apheresis (29). Our study showed that although the count of MNCs in apheresis products is decreased with high weight before transplantation, the number of WBCs, and neutrophils on apheresis day is directly related to the number of MNCs. In a study carried out by Moreb et al., being overweight and having a male gender increased the risk of poor mobilization (30).
It was found in our study that with an increase in the values of NDGI and TDIG, the number of CD34+ CPK and CD34+ cells in PAPBS decreased. The maximum number of CD34+ cells are seen in the peripheral blood when G-CSF is used for mobilization within 4-6 days after the time of injection; however, using G-CSF for more than 7 days has been shown to decrease the amount of progenitor cells (19). At doses greater than 5µg/kg/day, and 10µg/kg/day, the number of CD34+ cells in peripheral blood increases 7- and 28-fold, respectively (19). However, as shown in Figure 3, at high injection doses of G-CSF (TDIG >8.25, approximately 4000 µg), the number of CD34+ CPK and CD34+ cells in PAPBS are decreased. Zheng et al. showed that NDGI >5 days could be regarded as one of the negative factors of mobilization (31). It is possible that at high concentrations of G-CSF for a long time, the bone marrow undergoes hyperplasia, and mature WBCs are released into the bloodstream, reducing the number of HSCs (26). As shown in Figure 3, the number of WBCs increases in the peripheral blood on the day after apheresis with a logarithmic rise in TDIG >8.25. These cells are probably mature cells, and a possible reduction in the number of HSCs might ultimately result in a longer duration of engraftment at high TDIG levels. Therefore, based on previous studies, it is recommended to mobilize with G-CSF within 4-5 days (32). It was shown that the injection of 2.5×106/kg of CD34+ CPK with a short mobilization period will have a better transplantation outcome than the injection of 5×106/kg with a longer mobilization period (5). The standard dose of G-CSF is 5-16µg/kg/day, which is associated with a 38% failure in mobilization (5, 33). Increasing the dose of injectable G-CSF up to 40µg/kg/day is correlated with improved mobilization (34). The number of HSCs (CD34+) in the PAPBS sample should be evaluated, and G-CSF injection should be sustained for up to 4 days, according to the algorithms of various research. If the appropriate number of cells is not obtained, Plerixafor is injected for one day (5). Beartsch et al. identified platelet counts before mobilization as a predictor of the need for Plerixafor (35). Our study found a positive correlation among platelet counts on the day of apheresis and CD34+ CPK counts. Therefore, platelet count before apheresis can be suggested as a predictor of mobilization outcome.
Besides, larger-volume leukapheresis (LVL) can provide a useful yield for CD34+ CPK cells (36). In the patients with poor mobilization and CD34+ CPK<2×106/kg, the use of LVL may improve the count of CD34+ cells by 40-100 % (37). In our study, in parallel with an increase in the volume of blood processed during apheresis, the number of CD34+ CPK was increased; however, such an increment was not statistically significant.
Conclusion
The results of this study showed that the parameters of age, CD34+ CPK, and total dose of G-CSF are involved in the platelet and neutrophil engraftment, and they should be considered in the management of AHSCT process. For enhanced engraftment and AHSCT outcomes, decreasing the G-CSF injection days to <4 days and total dose of G-CSF on <4000 micrograms are suggested.
Acknowledgements
There is no financial support for this study; however, the authors would like to thank the staff of the BMT department of Taleghani Hospital, Tehran, for their general support.
Funding
None.
Authors' contributions
Mohammad Rafiee & Seyedeh Zahra Shahrokhi: Conceptualization, Data curation; Investigation; Writing and Project administration, Hossein Bonakchi: Formal analysis; Software, Sajjad Rahimi Pordanjani, Mehdi Allahbakhshian Farsani, Sina Mohagheghi: Review & editing, Mohammad Hossein Mohammadi: Conceptualization; Supervision and Validation.
Conflicts of Interest
The authors declare that they have no conflict of interest.
Received: 2023/03/23 | Accepted: 2023/06/26 | Published: 2023/10/29
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |



