BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://journal.zums.ac.ir/article-1-7430-en.html
2- Student Research Committee, Department of Neurology, School of Medicine, Zanjan University of Medical Sciences, Zanjan, Iran
3- School of Medicine, Zanjan University of Medical Sciences, Zanjan, Iran
4- Student Research Committee, Department of Neurology, School of Medicine, Zanjan University of Medical Sciences, Zanjan, Iran ,
Stroke is the greatest cause of mortality globally, with around 140,000 stroke fatalities and 800,000 new strokes occurring each year in the US alone (1). Indeed, many surviving patients have long-term disability and low quality of life. Patients with acute ischemic stroke (AIS) symptoms should receive intravenous tissue plasminogen activator (IV tPA) prior to four-and-a-half-hour window time with this class of medications, which includes alteplase, reteplase, and Tenecteplase, according to the most recent stroke therapy protocols and guidelines (2).
Among all the AIS incidences, nearly 14–29.6% are related to the wake-up stroke (WUS) and unknown time stroke. In WUS, the exact time of stroke incidence is unknown, and stroke can occur during sleep. Therefore, in the first evaluation, WUS treatment by tPA is forbidden because the incidence time is unknown (3). Despite all of the promising results of using tPA in WUS patients, the use of this drug in these patients is performed with caution. The defined guidelines for treating these patients have not been introduced, and there are no clear indications or contraindications. Indeed, tPA therapy in older people with AIS is crucial and requires more attention because of the increased risk of adverse effects. Previous studies have shown that the risk of ICH after the use of tPA increases by 4.9% in patients over 55 years of age and 10.3% in those over 75 years of age. Furthermore, additional research has shown that the incidence of ICH in people over 80 is 10%–13% higher than previously reported (4, 5). Therefore, tPA treatment at these ages is essential.
In this investigation, we thus assessed IV tPA in WUS patients at unknown periods to ascertain the safety and effectiveness of therapy based on the mismatch between DWI-FLAIR in MR images, and we followed up with patients to assess the treatment's effectiveness after three months.
2.1 Study Design and Setting
This study is a single-center, open-label clinical trial conducted at the Vali-e-Asr Hospital Stroke Care Unit (SCU) in Zanjan, Iran. The hospital has been recognized with multiple Diamonds State Awards by the Angle European Stroke Organization (ESO) and the Angle World Stroke Organization (WSO) between 2018 and 2023 and was designated a top center for intravenous thrombolysis (IVT) and acute phase protocols by SITS International in 2019, 2020, and 2022.
2.2 Participants
A total of 107 patients with wake-up stroke (WUS) were enrolled and divided into two groups: 53 patients who received intravenous tissue plasminogen activator (IV tPA) and 54 patients who did not receive thrombolysis. The inclusion criteria were acute neurological symptoms consistent with stroke upon awakening, presentation within four hours of symptom onset, age ≥18 years, and a diagnosis of acute ischemic stroke, confirmed as either wake-up stroke or of unknown onset.
The exclusion criteria included intracranial hemorrhage on CT or MRI, a modified Rankin scale (MRS) score >2 prior to stroke, contraindications to MRI, infarction exceeding one-third of the midbrain artery or more than 100 ml, conditions increasing bleeding risk (e.g., severe microangiopathy, thrombocytopenic purpura), age <18 years, pregnancy, recent stroke (within three months), history of symptomatic intracranial hemorrhage, use of anticoagulants or thrombolytic agents within 48 hours, platelet count <100,000, blood glucose levels <50 or >400 mg/dL, uncontrolled blood pressure, inherited or acquired hemorrhagic disorders, recent gastrointestinal or urinary bleeding, acute pancreatitis, severe liver disease, active bleeding in noncompressible areas, recent major surgery, head injuries, delays in MRI or treatment initiation, and absence of lesions in diffusion-weighted imaging (DWI).
2.3 Data collection
At admission, skilled neurologists evaluated the patients. Clinical evaluations comprised the MRS at discharge and three months after stroke, as well as the NIHSS at arrival and discharge. MRI sequences such as fluid-attenuated inversion recovery (FLAIR), SWI, T1-weighted imaging, diffusion-weighted imaging (DWI), and apparent diffusion coefficient (ADC) were used in imaging investigations. CT scans were conducted 24 hours following the injection of tPA or in the event of a headache, hypertensive crisis, or clinical deterioration. On average, it took 43.06 minutes from the start of symptoms until the injection of tPA.
2.4 Statistical analysis
SPSS version 26.0 (IBM, NY, USA) was used to analyze the data. Whereas categorical variables are given as proportions or frequencies, continuous variables are reported as means with standard deviations or medians with interquartile ranges based on data distribution. When necessary, statistical comparisons were made using the chi-square test, independent t test, paired t test, Mann-Whitney U test, and Fisher's exact test.
To evaluate the efficacy of tPA, binary logistic regression analysis was performed with the untreated tPA group as the reference category. The outcomes included functional independence, excellent functional outcomes, and mortality at discharge and three months. Ordinal logistic regression was used to assess the distribution of mRS scores at 90 days. All analyses were adjusted for baseline characteristics, including age, sex, ischemic heart disease, stroke history, diabetes mellitus, hypertension, atrial fibrillation, smoking, hyperlipidemia, and the NIHSS score. Odds ratios (ORs) and 95% confidence intervals (CIs) were reported, with statistical significance set at a p value of <0.05.
Various factors are evaluated in terms of clinical outcome (Table 2). The NIHSS score at admission was greater in the untreated group [12.5 (8–17.3) vs. 10 (5.5–15)], but the NIHSS score at discharge was significantly lower in the tPA-treated group. The discharge NIHSS score in the treated group was 4.51 (5.27), whereas it was 6.98 (3.75); in untreated group. Thus, the treatment had a greater impact on the patients (p=0.006).
In terms of the incidence of ICH, two patients in the tPA group experienced ICH, but none of the nontPA-treated patients experienced this side effect. However, a significant difference in the incidence of ICH as the main side effect was not found between tPA-treated and nontPA-treated patients (P=0.243).
In terms of mortality, in-hospital mortality was lower in the treated group, as in the treated group, two (3.8) patients died; however, in the untreated group, this number was 12 (22.2). Therefore, the treated group experienced significantly lower mortality (p=0.008). Indeed, after adjudication, the in-hospital mortality rate was considerably higher for untreated patients (OR = 6.56, 95% CI = 1.09–39.59; adjusted p = 0.040). (Table 3). Additionally, further analysis revealed a significantly lower total mortality rate (in-hospital + out-of-hospital mortality rate) in the treated group than in the control group [6 vs. 17 patients died (P=0.011)].
Regarding the mRS score, the results demonstrated that 49.1% of treated patients achieved favorable mRS scores at discharge. This number in the control group was reported to be 11.1% and was insignificant compared with that in the treated group. Therefore, at discharge, the group receiving the drug had a much better mRS score (p<0.001). Indeed, at the three-month follow-up, a comparison of the distribution of mRS scores at 90 days revealed a significant difference between two groups (P=0.011) (Figure 1). Interestingly, a substantial proportion of patients with mRS scores of 0–1 (indicating excellent function) and 0–2 (indicating functional independence) were detected in the treatment group (60.4% and 67.9%, respectively). The clinical outcomes of the groups are presented in Table 3. The untreated group had a lower proportion of excellent functional outcomes (mRS 0--1), with a significant difference after adjusting for baseline characteristics (OR = 0.24, 95% CI = 0.09--0.64; adjusted p = 0.004). The tPA-treated patients had mRS scores of 1.8 versus 3.2 in the untreated group, indicating good treatment results. Indeed, the excellent functional outcome three months later in the treated group was 60.4%, which was better than that in the untreated group (p = 0.001). Therefore, the discharge outcome and three-month follow-up were better in the treated group.
Furthermore, 20 patients were not treated with tPA and 21 patients were treated with it, according to the analysis of patients over 80 (Table 4). The outcomes and side effects of the treated and untreated groups above the age of 80 did not differ significantly.
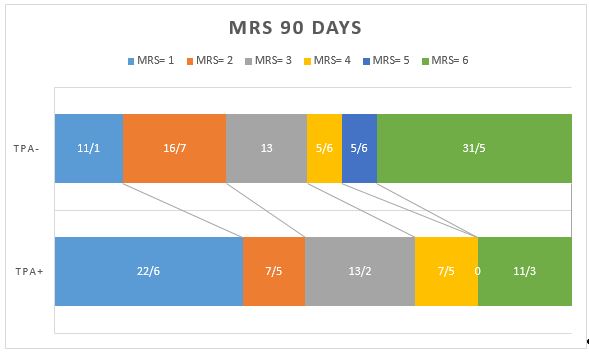
Figure 1. Comparison of the mRS score 90 days later in the group receiving venous thrombolysis. (tPA+) with the group not receiving the drug (tPA-).
Table 1. Baseline characteristics of wake-up stroke patients.
| Variables | tPA+ (N =53) |
tPA- (N =54) |
P value |
| Age, years [median (IQR)] | 75(63-83) | 75(62.75-84) | 0.217 |
| Gender, male [N (%)] | 26(49.1) | 23(42.6) | 0.563 |
| Ischemic heart disease [N (%)] | 11(20.8) | 10(18.5) | 0.812 |
| History of stroke [N (%)] | 9(17) | 15(27.8) | 0.228 |
| Diabetes mellitus [N (%)] | 13(24.5) | 20(37) | 0.210 |
| Hypertension [N (%)] | 42(79.2) | 47(87) | 0.312 |
| History of Atrial Fibrillation [N (%)] | 5(9.4) | 9(16.7) | 0.391 |
| Opium [N (%)] | 0(0) | 0(0) | N/A |
| Smoking [N (%)] | 11(20.8) | 7(13) | 0.312 |
| Hyperlipidemia [N (%)] | 5(9.4) | 5(9.3) | 1.000 |
| Systolic blood pressure, mmHg [mean (SD)] | 158.83(29.11) | 152.48(22.77) | 0.212 |
| Diastolic blood pressure, mmHg [median (IQR)] | 90(80-95) | 90(80-90) | 0.636 |
| Blood sugar, mg/dl [median (IQR)] | 114(98-158) | 135.5(106-189.3) | 0.217 |
| NIHSS score [median (IQR)] | 10(5.5-15) | 12.5(8-17.3) | 0.013 |
| Days of hospitalization [median (IQR)] | 5(3-11) | 5(3-7) | 0.221 |
tPA+/-, tissue plasminogen activator receiver or not; NIHSS, National Institutes of Health Stroke Scale.
The P value indicates a comparison between groups.
Table 2. Efficacy and safety outcomes in two groups of wake-up stroke patients.
| Outcomes | tPA+ (N =53) |
tPA- (N =54) |
P value | |||
| mRS 0-1 at 90 days [N (%)] | 32(60.4) | 15(27.8) | 0.001 | |||
| mRS 0-2 at 90 days [N (%)] | 36(67.9) | 24(44.4) | 0.019 | |||
| mRS distribution at 90 days | ||||||
| 0 [N (%)] | 20(37.7) | 9(16.7) | 0.011 | |||
| 1 [N (%)] | 12(22.6) | 6(11.1) | ||||
| 2 [N (%)] | 4(7.5) | 9(16.7) | ||||
| 3 [N (%)] | 7(13.2) | 7(13) | ||||
| 4 [N (%)] | 4(7.5) | 3(5.6) | ||||
| 5 [N (%)] | 0(0) | 3(5.6) | ||||
| 6 [N (%)] | 6(11.3) | 17(31.5) | ||||
| ICH [N (%)] | 2(3.8) | 0(0) | 0.243 | |||
| NIHSS score discharge [mean (SD)] | 4.51(5.21) | 6.98(3.75) | 0.006 | |||
| In-hospital death [N (%)] | 2(3.8) | 12(22.2) | 0.008 | |||
| Out-of-hospital death [N (%)] | 4(7.5) | 5(9.3) | 1.000 | |||
| Total death [N (%)] | 0.011 |
Table 3. Efficacy and safety outcomes between two groups of wake-up stroke patients.
| Outcomes | tPA+ (N =53) |
tPA- (N =54) |
Unadjusted | Adjusted | ||
| OR (95% CI) | P value | OR (95% CI) | P value | |||
| mRS 0-1 at 90 days [N (%)] | 32(60.4) | 15(27.8) | 0.25(0.11-0.57) | 0.001 | 0.24(0.09-0.64) | 0.004 |
| mRS 0-2 at 90 days [N (%)] | 36(67.9) | 24(44.4) | 0.38(0.17-0.83) | 0.015 | 0.41(0.16-1.05) | 0.062 |
| ICH [N (%)] | 2(3.8) | 0(0) | N/A | N/A | N/A | N/A |
| In-hospital death [N (%)] | 2(3.8) | 12(22.2) | 7.29(1.54-34.38) | 0.012 | 6.56(1.09-39.59) | 0.040 |
| Out-of-hospital death [N (%)] | 4(7.5) | 5(9.3) | 1.25(0.32-4.94) | 0.750 | 0.89(0.19-4.17) | 0.883 |
| Total death [N (%)] | 6(11.3) | 17(31.5) | 0.28(0.1-0.78) | 0.014 | 0.3(0.09-1.03) | 0.056 |
Table 4. Efficacy and safety outcomes in two groups of wake-up stroke patients older than 80 years.
| Outcomes | tPA+ (N =21) |
tPA- (N =20) |
P value | |||
| mRS 0-1 at 90 days [N (%)] | 10(47.6) | 4(20) | 0.062 | |||
| mRS 0-2 at 90 days [N (%)] | 11(52.4) | 6(30) | 0.146 | |||
| mRS distribution at 90 days | ||||||
| 0 [N (%)] | 6(28.6) | 2(10) | 0.380 | |||
| 1 [N (%)] | 4(19) | 2(10) | ||||
| 2 [N (%)] | 1(4.8) | 2(10) | ||||
| 3 [N (%)] | 5(23.8) | 3(15) | ||||
| 4 [N (%)] | 1(4.8) | 1(5) | ||||
| 5 [N (%)] | 0(0) | 1(5) | ||||
| 6 [N (%)] | 4(19) | 9(45) | ||||
| ICH [N (%)] | 2(9.5) | 0(0) | 0.157 | |||
| NIHSS score discharge [mean (SD)] | 5.24 ± 6.12 | 6.75 ± 2.75 | 0.312 | |||
| In-hospital death [N (%)] | 2(9.5) | 7(35) | 0.067 | |||
| Out-of-hospital death [N (%)] | 2(9.5) | 2(10) | 0.959 | |||
| Total death [N (%)] | 4(19) | 9(45) | 0.074 |
Discussion
The result of this study, comparing the efficacy of Agomelatine and Sertraline in treating depression symptoms in HF patients over the twelve-week follow-up, showed a reduction in depression symptoms in both treatment groups during twelve weeks based on the BDI-II and HAM-D. Both Agomelatine and Sertraline were effective, with no significant difference observed between the two medications.
Conclusion
Hysteroscopic myomectomy for submucosal myoma in infertile patients is a safe procedure without major complications.
Declarations
Acknowledgements
We would like to express our sincere gratitude to everyone who contributed to this study. We are especially thankful to Babol University of Medical Sciences for their financial support.
Ethical Considerations
Under the Ethics Committee of Babol University of Medical Sciences, the study was approved (Approval ID: IR.MUBABOL.REC.141138) and registered in the Iranian Registry of Clinical Trials (Registration Number: IRCT20170606034348N7). All participants signed an informed consent form following detailed explanations of the study's objectives and procedures.
Authors' Contributions
N.Z. was involved in data collection, data analysis, manuscript writing, and editing; A.H., A.R., S.A., H.SH, H.H., and N.T. contributed to project development, data analysis, and editing; and R.H. participated in project development, data analysis, manuscript writing, and editing. All authors approved the submitted version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest regarding the publication of this study.
Fund or Financial Support
We sincerely thank Babol University of Medical Sciences for their financial support.
Using Artificial Intelligence Tools (AI Tools)
The authors were not utilized AI Tools.
Received: 2024/10/22 | Accepted: 2025/02/11 | Published: 2025/03/13
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |







