BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://journal.zums.ac.ir/article-1-7506-en.html


 , Parastoo Amiri2
, Parastoo Amiri2 

 , Ali Khatib3
, Ali Khatib3 

 , Mohammad Amin Ebrahimi1
, Mohammad Amin Ebrahimi1 

 , Shamim Shahrestani1
, Shamim Shahrestani1 

 , Fereshteh Ghorat *4
, Fereshteh Ghorat *4 


2- Non-Communicable Diseases Research Center, Sabzevar University of Medical Sciences, Sabzevar, Iran
3- Department of Nursing, School of Nursing and Midwifery, Neyshabur University of Medical Sciences, Neyshabur, Iran
4- Non-Communicable Diseases Research Center, Sabzevar University of Medical Sciences, Sabzevar, Iran ,
Osteoarthritis (OA), also known as degenerative arthritis, is the most common joint disorder globally, marked by progressive degeneration of articular cartilage, subchondral bone remodeling, and synovial inflammation (1). This condition affects approximately 630 million individuals worldwide and has witnessed a 113% increase in prevalence over the past three decades (2). Epidemiological data indicate that OA disproportionately impacts older adults, with 18% of women and 9.6% of men aged 60 and above experiencing this debilitating disease (3, 4).
The primary symptoms of knee OA include persistent joint pain, stiffness, instability, reduced mobility, and crepitus (5). Additionally, patients frequently report non-specific complaints such as fatigue, depression, lethargy, and joint swelling, which overlap with symptoms of other chronic conditions such as systemic lupus erythematosus and rheumatoid arthritis (6). Among these, pain remains the most significant and persistent concern, often worsening as the disease progresses. Despite extensive research, no definitive cure for OA exists, emphasizing the need for interventions aimed at symptom relief and functional improvement (7, 8).
Current treatment approaches for OA range from symptom-focused pharmacological therapies, such as acetaminophen and non-steroidal anti-inflammatory drugs (NSAIDs), to non-pharmacological methods, including physical therapy, hot or cold packs, and exercise (9-11). However, systemic NSAID use is frequently associated with adverse effects, such as gastrointestinal disturbances, hepatotoxicity, and nephrotoxicity. Topical NSAIDs, such as diclofenac, offer localized pain relief and reduced systemic side effects but are not devoid of complications (12).
Given these limitations, there is growing interest in complementary and alternative medicine for managing pain and OA, with a preference for natural and minimally invasive treatments (13, 14). Alpinia galanga, a member of the ginger family (Zingiberaceae), has long been recognized in traditional medicine for its potent anti-inflammatory and analgesic properties (15). Native to South and Southeast Asia, this perennial plant contains bioactive compounds such as methyl cinnamate and camphor, which contribute to its diverse therapeutic effects, including pain relief, anti-inflammatory action, and inhibition of prostaglandin biosynthesis (16-19).
Although Alpinia galanga has demonstrated efficacy in managing various conditions such as hyperglycemia, dysentery, and neural protection, its potential role in treating knee OA remains underexplored. This study aims to evaluate the efficacy of topical Alpinia galanga oil in alleviating OA symptoms and to compare its effects with the well-established diclofenac gel.
2.1 Study Design and participants
The current clinical trial was accomplished between December 2021 and May 2022 on clients at health centers in Sabzevar, northeastern Iran. The study recruited 160 patients who sought treatment at Sabzevar health centers.
The inclusion criteria were as follows: having diagnosis of osteoarthritis (OA) confirmed by the American College of Rheumatology criteria with the patient’s condition being graded 1 to 3 according to the Kellgren-Lawrence classification system; age range 45-70 years. On the other hand, the exclusion criteria were: abnormal knee anatomy or previous joint arthroplasty surgery, the presence of knee joint infections, serious underlying diseases such as hepatic or renal conditions, significant dermal conditions affecting the knee area, intra-articular steroid injections within three months prior to the study, intramuscular steroid injections within one month prior to the study, intolerance or sensitivity to diclofenac or Alpinia galanga oil, and the use of narcotics, psychotropic medications, or corticosteroids.
2.2 Intervention
The Ethics Committee in Human Research at Sabzevar University of Medical Sciences granted approval for the research proposal, assigning it the ethics code IR.MEDSAB.REC.1398.124 and IRCT code IRCT20200701047978N1. Prior to their participation, all subjects provided written informed consent. Participants were then randomly assigned to either the intervention group or the control group using balanced block randomization. In the intervention group (n=81), the patients applied 5–10 drops of Alpinia galanga oil topically to the affected joint twice daily for 28 days. The oil was massaged into the joint for two minutes during each application. In the control group (n=77), the patients applied 1% diclofenac gel (Behvazan Pharmaceutical Company, 60 g) following the same application protocol. The participants were advised to maintain their current medication regimen without modifications. In cases of persistent pain, acetaminophen was permitted as an adjunct. Balanced block randomization was implemented using a computer-generated random sequence provided by a statistical consultant. The study was triple-blinded to minimize bias:
-
Both treatments were packaged in identical containers labeled as "Box A" or "Box B" to ensure blinding of participants and researchers.
-
Data collection, patient evaluation, and form completion were performed by the project director and an assistant, both blinded to the intervention group.
-
Data analysis was conducted by a statistician and project consultant, also blinded to group assignments.
Procedure for Preparing Alpinia galanga Oil and Placebo. The Alpinia galanga oil was processed and prepared at School of Pharmacy, Tehran. To this aim, 100 g of powdered Alpinia galanga roots was macerated in 600 mL of 25% ethanol for 24 h with continuous agitation to obtain the hydro alcoholic extract. The prepared extract was then mixed with 800 g of cold-pressed sesame oil, with the resulting mixture heated until all water content was evaporated. The placebo involved only cold-pressed sesame oil.
2.3 Outcome Measures
The primary outcomes were pain intensity, physical dysfunction, and joint stiffness, assessed using the Western Ontario and McMaster Universities Arthritis Index (WOMAC) questionnaire. The WOMAC questionnaire evaluates three domains: Pain intensity, Physical dysfunction, Joint stiffness. Each domain was scored on a scale of 0–4, with higher scores indicating worse outcomes. Assessments were conducted at baseline, day 14, and day 28 post-treatment.
2.4 Statistical Analysis
To assess the normality of data distribution, researchers utilized the Kolmogorov-Smirnov test. Independent t-tests were conducted to compare groups, while ANOVA was used for analyzing repeated measures. The statistical analysis was performed using SPSS version 16, and results were considered statistically significant when P<0.05.
Baseline comparisons using independent t-tests revealed no significant differences between groups in pain intensity, joint stiffness, motor function, exercise capacity, or quality of life (Table 2). Both treatment groups demonstrated significant improvements in all evaluated outcomes during the study period (Table 3).
Pain Intensity: Paired t-tests showed significant reductions in pain scores in both groups over 21 days (P < 0.001), with no statistically significant difference between them (P = 0.829, ANOVA). Motor Function: Motor function improved significantly in both groups (P < 0.001), with no significant difference between them (P = 0.155, ANOVA). Joint Stiffness: Stiffness diminished significantly in both groups (P < 0.001), with no significant intergroup difference (P = 0.772, ANOVA). Exercise Capacity: Exercise scores improved significantly in both groups (P < 0.001), with no significant intergroup difference (P = 0.909, ANOVA).
Quality of Life: Quality of life scores improved significantly in the Alpinia galanga group (P < 0.001). The diclofenac group showed no statistically significant improvement in this measure (P = 0.139, paired t-test).
While both treatments led to significant improvements across all outcome measures, no significant differences were observed between the groups in terms of pain, motor function, stiffness, or exercise capacity. However, the Alpinia galanga group showed a significant improvement in quality of life, unlike the diclofenac group. In the current study, no complications were observed in either the Alpinia galanga group or diclofenac group.
Table 1. Demographic characteristics of patients treated with Alpinia Galanga oil and diclofenac gel
| Group Variable mean (SD) |
Alpinia Galanga oil N=81 |
Diclofenac gel N=77 |
| Age | 62.56 (7.52) | 61.66 (7.72) |
| Gender (female/male) f (%) | 66 (81.5); 53 (68.8) | 53(68.8); 24(31.2) |
| Education (primary/high school) | 66(81.5); 15(18.5) | 68(88.3); 9(11.7) |
| BMI | 25.88(2.48) | 26.38(2.55) |
Table 2. Comparison of pain intensity index, joint stiffness, motor function, exercise, and quality of life in both groups before the intervention
| Variable | Alpinia Galanga oil group N=81 |
Diclofenac gel group n=77 |
P-value* |
| Pain intensity | 51.60±18.60 | 52.88±20.47 | 0.467 |
| Joint stiffness | 68.18±20.29 | 69.28±18.94 | 0.727 |
| Motor function | 57.68±21.30 | 52.88±21.80 | 0.164 |
| Exercise | 35.06±30.11 | 35.71±22.32 | 0.878 |
| Quality of life | 49.99±16.6 | 47.79±19.08 | 0.780 |
Table 3. Comparison of patients treated with Alpinia Galanga oil and diclofenac gel at specified times
| Alpinia Galanga oil N=81 |
Diclofenac gel N=77 |
||||||||
| Variable | Week 0 | Week 2 | Week 3 | Paired T-test | Week 0 | Week 2 | Week 3 | Paired T-tedt | P (ANOVA) |
| Pain intensity | 50.61±18.60 | 57.12±17.97 | 59.91±18.62 | 0.001 | 52.88±20.47 | 57.90±19.05 | 58.80±19.90 | 0.001 | 0.829 |
| Motor function | 57.68±21.30 | 61.93±20.10 | 63.64±20.71 | 0.001 | 52.88±21.80 | 57.60±21.01 | 58.60±21.20 | 0.001 | 0.155 |
| Joint stiffness | 68.18±20.29 | 73.97±18.07 | 76.90±17.87 | 0.001 | 69.28±18.94 | 73.09±18.40 | 74.13±19.88 | 0.001 | 0.772 |
| Exercise | 64.92±30.11 | 36.97±30.30 | 26.97±30.15 | 0.001 | 64.28±22.22 | 36.81±22.37 | 27.92±21.67 | 0.001 | 0.909 |
| Quality of life | 46.99±16.66 | 48.64±16.68 | 50.20±17.35 | 0.001 | 47.79±19.80 | 48.48±19.39 | 48.57±19.18 | 0.139 | 0.907 |
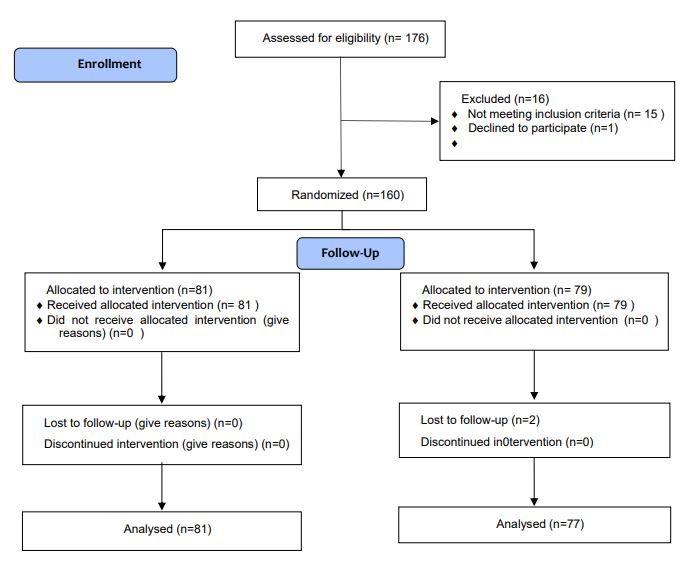
Figure 1. CONSORT Diagram of Study
Discussion
Osteoarthritis (OA) is the most prevalent joint disease, primarily characterized by the localized degeneration of joint cartilage as well as associated structural and functional impairments (20, 21). To mitigate the systemic side effects of oral and parenteral treatments, topical therapies are increasingly utilized, particularly for chronic conditions such as OA that require long-term management. The goal of these interventions is to provide effective symptom relief with minimal adverse effects (22, 23).
This study evaluated the effects of topical Alpinia galanga oil on knee OA symptoms and compared its efficacy with diclofenac gel. Among the 160 participants, both treatments significantly reduced pain intensity, joint stiffness, and motor dysfunction over the intervention period. Improvements in exercise capacity were also observed in both groups. Importantly, there were no statistically significant differences between the two treatments in any of the measured outcomes, including quality of life, indicating comparable efficacy.
These findings are consistent with prior studies. For example, Syahruddin et al. (2017) examined the effects of Kaempferia galanga (a close relative of Alpinia galanga L.) oral extract on knee OA symptoms and reported similar improvements in pain, stiffness, and function, comparable to meloxicam. While their study utilized oral administration, the outcomes align closely with our findings, suggesting that Alpinia galanga may have broad therapeutic potential across different delivery methods (20).
Similarly, Phitak et al. (2009) identified Alpinia galanga as a promising therapeutic agent for OA, highlighting its potential for pain relief and cartilage protection. Furthermore, its analgesic and anti-inflammatory effects are well-supported by related studies on ginger, another member of the Zingiberaceae family (24). For instance, studies by Yip and Tam, Bidal et al., as well as Niempoog et al. demonstrated the efficacy of ginger in alleviating OA symptoms, underscoring the potential of Alpinia galanga as an effective alternative to conventional treatments (25-30).
In contrast to NSAIDs, which are known to inhibit cartilage matrix synthesis and potentially accelerate cartilage degeneration, Alpinia galanga offers a safer profile. Its bioactive compounds, including methyl cinnamate, galangulf, alpinin, and camphor, contribute to its anti-inflammatory and analgesic properties, making it a viable option for long-term OA management (31, 32). However, it is crucial to note that the available research on Alpinia galanga is limited, and more comprehensive studies are required to confirm its safety and efficacy.
Although this study demonstrated the potential of Alpinia galanga oil as an effective treatment for OA, the lack of long-term data and high-quality trials limits the generalizability of the findings. Subsequent studies should prioritize conducting larger, multi-site clinical trials with longer follow-up durations to confirm the persistence of the effects and explore the underlying mechanisms through which the intervention operates.
Conclusion
The present study suggested that topical application of Alpinia galanga oil is as efficacious as diclofenac gel in relieving the symptoms of knee osteoarthritis, such as pain severity, motor dysfunction, joint rigidity, and exercise potential. Both treatments resulted in significant improvements during the intervention, and no statistically significant disparity was noted between the two groups with respect to these findings. Furthermore, contrary to diclofenac gel, the use of Alpinia galanga oil was accompanied by a significant enhancement in quality of life. Considering the comparable efficiency and natural origin, Alpinia galanga oil brings about an optimistic choice for the amelioration of knee osteoarthritis symptoms, specifically in patients who look for herbal and complementary therapies. Future studies, including long-term studies and multi-centered trials, are recommended to approve its safety and therapeutic capacity.
Declarations
Acknowledgements
Our gratitude extends to the elderly patients who participated in this study, the dedicated staff at Sabzevar's health centers, and the research and technology deputy of Sabzevar University of Medical Sciences for their valuable cooperation.
Ethical Considerations
University of Medical Sciences granted approval for this research, assigning the ethics code IR.MEDSAB.REC.1398.124 and the clinical trial code IRCT20200701047978N1. Additionally, all study participants provided informed consent throughout the course of the investigation.
Authors' Contributions
Samaneh Sadat Asadi contributed to designing project, initial studies, as well as data extraction and collection; Parastoo Amiri contributed to statistical analysis; Ali Khatib (co-researcher) contributed to preliminary studies and writing the first draft; Mohammad Amin Ebrahimi and Shamim Shahrestani (co-researchers) participated in preliminary studies and writing the manuscript. Fereshteh Ghorat (principal researcher) was responsible for correspondence, participation in preliminary studies, compilation of different parts of the research project, and scientific editing of the paper.The final manuscript was read and approved by all of the authors.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
Fund or Financial Support
Sabzevar University of Medical Sciences was the financial supporter of this project.
Using Artificial Intelligence Tools (AI Tools)
The authors were not utilized AI Tools.
Received: 2024/09/3 | Accepted: 2025/01/12 | Published: 2025/03/13
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |




