BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://journal.zums.ac.ir/article-1-6080-en.html
2- Dept. of Endocrinology and Metabolism, Faculty of Medicine, Tehran University of Medical Sciences, Tehran, Iran
✅ According to our findings, PLP imposed a beneficial effect on vascular complications in atherosclerotic rats, which could be attributed to its antioxidant and anti-inflammatory properties. Moreover, PLP has positive impacts on insulin function, dyslipidemia, and GLO system activity.
Atherosclerosis and nephropathy are common vascular complications of diabetes and are considered the leading cause of death in the world (1, 2). According to the literature, oxidative stress and inflammation induce insulin resistance and vice versa (3). Furthermore, insulin resistance, even in the absence of hyperglycemia, is an important factor in the pathogenesis of vascular complications (4, 5). Insulin resistance evokes inflammation and dyslipidemia along with interfering with insulin signaling in vascular cells, which occurs during atherosclerosis progress (5). Moreover, oxidative stress increases the level of transforming growth factor-β1 (TGF-β1) in the kidney as an important factor in nephropathy (6).
Di-carbonyl compounds, such as methylglyoxal (MGO) have been known to lead to oxidative stress and inflammation contributing to a reduction in insulin secretion, insulin resistance, and vascular complications (7, 8). The diminished activity of the glyoxalase (GLO) system, as the main defense against MGO accumulation, in the presence and absence of hyperglycemia plays role in insulin dysfunction, atherosclerosis (9), and nephropathy (10). Therefore, the induction of GLO system activity has recently become a major therapeutic target in vascular complications (11).
Preventive medicine is suggested to be the best approach for managing vascular complications (12). We recently investigated the favorable effect of some natural products on diabetic vascular complications (13-15). Pyridoxal phosphate (PLP), the active form of vitamin B6, is an essential coenzyme in numerous metabolic reactions (16) and might play a crucial role in protecting cells against oxidative stress. Some evidence exhibited that the deficiency of vitamin B6 along with variations in its metabolism contribute to vascular disorders due to the induction of endothelial dysfunction, oxidative stress, and inflammation (16, 17). Moreover, low circulating PLP is thought to be a possible indicator of inflammatory status and serum PLP is significantly correlated with the severity of coronary atherosclerosis (18).
With this background in mind, we investigated the impact of PLP on the formation of atheromatous plaque and renal function in atherosclerotic rats. Furthermore, the inhibitory effect of PLP on the generation of LDL oxidation products under in vivo and in vitro conditions was studied.
Materials
In this experimental study, all materials with analytical grade were purchased from Sigma and Merck Chemical Companies, Germany.
In Vivo Studies
Animals
Nine-week-old male Wistar rats weighing 185±15 g were purchased from Pasteur Institute of Iran, Karaj. Animals were housed under controlled conditions with free access to food and water. After two weeks, they were divided into the two main groups of normal and atherosclerotic. Atherosclerosis was induced in rats with an atherogenic diet. Afterwards, each of the two main groups was divided into two subgroups of 10 rats. The normal groups were fed a standard chow diet, while the atherosclerotic groups were fed an atherogenic diet (a chow diet containing 1% cholesterol and 0.5% cholic acid).
The subgroups were named as “N” for the normal group and “A” for the atherosclerotic group with no more treatments. The two similar subgroups under PLP treatment entailed N (PLP) and A (PLP), which received 0.18% PLP daily in their drinking water for three months. The experiment protocol was approved with the code of IR.ARUMS.REC.1397-176 by Animal Ethics Committee in accordance with the guidelines for the care and use of laboratory animals prepared by Ardabil University of medical sciences.
At the end of the intervention, fasting blood samples were collected from the hearts of the rats and were then transferred to test tubes with and without EDTA. Serum samples were obtained by 10 min centrifugation of blood at 1500 g (4°C) and were stored at −70°C until further measurements. The aortas and kidneys of study rats were dissected and weighed immediately.
Biochemical Parameters
Fasting blood sugar (FBS), triglyceride (TG) (19), total cholesterol (TC), LDL, and high-density lipoprotein (HDL) were measured by enzymatic colorimetric methods. The LDL/HDL ratio was used to determine the atherogenic index. Serum insulin levels were determined using enzyme-linked immunosorbent assay (ELISA) using a rat insulin kit (Mercodia, Uppsala, Sweden). Moreover, a homeostasis model assessment of insulin resistance (HOMA-IR) was calculated.
Methylglyoxal
Serum MGO was measured utilizing reverse-phase high-performance liquid chromatography (HPLC) as explained previously (20).
Oxidative and Inflammatory Markers
Advanced oxidation protein products (AOPPs) as oxidative markers were assessed utilizing spectrophotometric detection according to the method of Witko-Sarsat et al. as described in our previous paper (20). Inflammatory markers, namely high-sensitivity C-reactive protein (hs-CRP) and TGF-β1 were measured using CUSABIO ELISA kits (Wuhan, Hubei, China).
LDL Oxidation Products
Conjugated dienes (CDs) in LDL lipids, as early products of LDL oxidation, were analyzed spectrometrically at 234 nm. The fluorescence intensity of the end LDL oxidation product, named as oxidation fluorescent product (OFP), was recorded at the maximum emission of 430 nm upon excitation at 360 nm (20).
Enzymatic Assay
The activity of glyoxalase-1and 2 (Glo-I and Glo-II) in hemolysate was determined by measuring the rates of initial formation and hydrolysis of S-D-lactoylglutathione and enzymes activity (20).
Pathologic Study
The entire aortas of all rats from all groups were collected and fixed in 10% buffered formalin. Next, sections were prepared from each segment and were stained with hematoxylin and eosin (H&E) stains for pathological examination.
In Vitro Formation of LDL Oxidation Products
LDL Extraction and Oxidation
The LDL was extracted from serum specimens and was incubated with CuSO4 in the absence and presence of 0.18% PLP. The procedure of extraction and incubation was similar to what was explained previously (21). Briefly, LDL was isolated from the sera of normal rats with sodium heparin and was incubated in the presence and absence of CuSO4 (10 mmol/L) and PLP (15 mmol/L).
Oxidized LDL Products
The resistance of LDL against oxidative modification with CuSO4, with and without PLP, was determined photometrically at 234 nm (22). In addition, the FOP of LDL was measured by the method explained in the “LDL Oxidation Products” section.
Statistical Analysis
Descriptive analysis of all data was expressed as mean±standard deviation (SD). Multivariate analysis of variance (MANOVA) test with Tukey posthoc test was used to compare the different variables assessed in all four groups utilizing the SPSS 16 (SPSS Inc., Chicago, IL., USA). Statistical significance was considered as
P-value< 0.05.
In Vivo Experiments
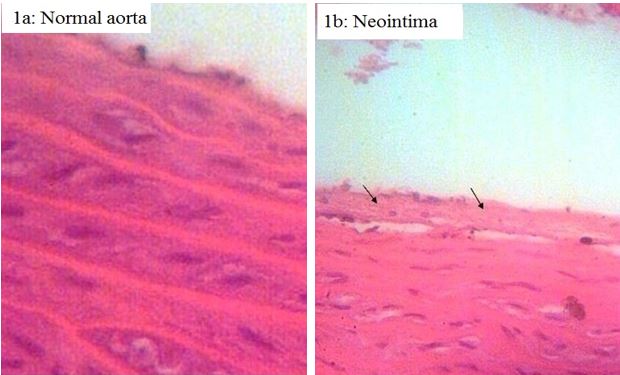
The impacts of PLP administration on the formation of aortic lesions in normal and atherosclerotic rats are shown in Figures 1a-1b. Neointima (Figure 1b) and aorta plaque were found only in the atherosclerotic rats. However, the vitamin interfered with the generation of any aortic lesions. Furthermore, the histological observations in the aorta of the treated atherosclerotic rats were similar to those of the untreated and treated normal groups (Figure 1a).
Figure 1. Effect of pyridoxal phosphate on atheromatous plaque formation in normal (N) and atherosclerotic (A) rats (stained by H&E & original magnification ×200). (a) Normal aorta in N, N (PLP) and A (PLP) groups. (b) Formation of neointima in A group. (c) Atherosclerosis plaque formation in A group
Table 1 represents the results of FBS, insulin, HOMA-IR, lipid profile, and kidney dysfunction tests in all groups. Insulin, HOMA index (as a marker of insulin resistance), TG, TC, LDL, and the atherogenic index increased significantly due to atherosclerosis induction. Our findings demonstrated that PLP compensated for these changes and decreased the levels of TC and TG in the treated, normal, and atherosclerotic groups. Furthermore, Cr, PU, and kidney weight index (as kidney dysfunction tests), as well as TGF-β1 (Table 2) were lower in the treated atherosclerotic group than in the untreated group (P<0.001).
Results of comparing oxidative markers, inflammatory factors, the activity of the GLO system, and MGO levels between all groups are summarized in Table 2. Reductions in the AOPP, LDL oxidation products, and hs-CRP of the atherosclerotic rats reveals that the treatment exerted antioxidant and anti-inflammatory influences (P<0.001). Furthermore, PLP induced Glo-I and Glo-II activity and diminished MGO levels in the mentioned groups (P<0.001).
Table 1. The effect of pyridoxal phosphate (PLP) on FBS, insulin, HOMA-IR, lipid profile, and kidney function tests in normal (N) and atherosclerotic (A) rats.
| Parameter | Groups | |||
|---|---|---|---|---|
| N | N (PLP) | A | A (PLP) | |
| Fasting blood sugar (mmol/L) | 4.49 ± 0.26 | 3.97 bb b ± 0.29 | 4.89 ± 0.47* | 4.66 ± 0.4 *, # |
| Insulin (µU/mL) | 17.91 ± 1.36 | 18.53± 0.67 | 26.84 ± 0.60 * | 21.89 ± 0.55 *, # |
| HOMA-IR | 3.57 ± 0.15 | 3.26 ± 0.17 | 5.83 ± 0.18 * | 4.53 ± 0.16 *, # |
| Triglyceride (mmol/L) | 0.93 ± 0.03 | 0.78 ± 0.02 *, | 2.83 ± 0.08 * | 1.02 ± 0.03 *, # |
| Total cholesterol (mmol/L) | 2.22 ± 0.07 | 1.99 ± 0.09 *, # | 6.42 ± 0.16 * | 4.44 ± 0.13 *, # |
| HDL (mmol/L) | 1.30 ± 0.05 | 1.25 ± 0.05 | 0.43 ± 0.01 * | 0.78 ± 0.02 *, # |
| LDL (mmol/L) | 0.43 ± 0.02 | 0.33 ± 0.01 | 4.68 ± 0.12 * | 3.20 ± 0.06 *, # |
| HDL/LDL | 0.33 ± 0.01 | 0.27 ± 0.01 *, # | 11.00 ± 0.21 * | 4.15 ± 0.11 *, # |
| Creatinine (µmol/L) | 61.50± 4.24 | 57.33 ± 1.86 | 96.50± 5.74 * | 71.00 ± 2.60 *, # |
| Proteinuria (mg/24 h) | 13.00 ± 0.57 | 10.87 ± 0.40 | 347.50 ± 17.50 * | 170.00 ± 8.11 *, # |
| Kidney weight index (g/100g body weight) | 0.86 ± 0.06 | 0.87 ± 0.05 | 1.23 ± 0.09 * | 0.98 ± 0.08 *, # |
* Indicates significance of data comparing group N with other groups (P<0.001)
# Indicates significance of data comparing group A with other groups (P<0.001)
Table 2. Effect of pyridoxal phosphate (PLP) on glyoxalase activity and methylglyoxal level as well as oxidative stress and inflammatory markers of the normal (N) and atherosclerotic (A) Rats.
| Parameter | Groups | |||
|---|---|---|---|---|
| N | N (PLP) | A | A (PLP) | |
| Methylglyoxal (µmol/L) | 15.50 ± 1.60 | 13.12 ± 0.63 | 93.00 ± 5.10 * | 48.60 ± 2.30 *, # |
| Glyoxalase-I (U/mL) | 32.64 ± 1.89.90 | 41.03 ± 2.33 *, # | 19.91 ± 0.95 * | 30.75 ± 2.01 *, # |
| Glyoxalase-II (U/mL) | 33.42 ± 1.96 | 42.65 ± 2.76 *, # | 21.53 ± 1.20 * | 28.63 ± 1.97 *, # |
| Early oxidation products of LDL (µmol/L) |
13.81± 0.89 | 12.95 ± 0.75 *, # | 108.33 ± 5.45 * | 67.61 ± 5.67 *, # |
| End oxidation products of LDL (µmol/L) |
248.73 ± 12.33 | 227.65 ± 10.05 *,# | 514.83 ± 27.30 * | 432.37 ± 22.97 *, # |
| Advanced oxidation protein products (µmol/L) | 28.80 ± 1.02 | 18.82 ± 0.85 *, # | 89.14 ± 3.23 * | 61.83 ± 2.84 *, # |
| hs CRP (ng/mL) | 235.02± 10.23 | 187.15 ± 7.55 *, # | 596.77± 25.21 * | 400.23 ± 17.81 *, # |
| TGF-β1 (pg/mL) | 26.32± 1.43 | 23.89 ± 1.09 | 87.23± 6.20* | 56.46 ± 3.62 *, # |
* Indicates significance of data comparing group N with other groups (P<0.001)
# Indicates significance of data comparing group A with other groups (P<0.001)
In Vitro Study
The levels of early and end LDL oxidation products (Table 3) decreased following the treatment.
Table 3. The effect of pyridoxal phosphate (PLP) on the formation of LDL oxidation products in the test tube experiment
| Tube Content | Early oxidation products (µmol/L) | End Oxidation products (AU) |
| LDL | 16.94±0.57 | 15.17±0.44 |
| LDL+ CuSO4 | 135.56±4.55 | 500.01±10.84 |
| LDL+ CuSO4 + PLP | 50.83±1.22 | 110.01±2.36 |
Discussion
According to the results of the current study, PLP reduced the levels of the markers of oxidative stress, carbonyl stress, and inflammation in atherosclerotic rats. It could be concluded that PLP prevented the formation of atheromatous lesions and improved kidney function in these animals. Furthermore, PLP had a corrective effect on insulin resistance, dyslipidemia, and the activity of the GLO system. Moreover, the inhibitory effect of the treatment on in vitro LDL oxidation confirms the obtained results.
The PLP was shown to impose positive effects on microvascular and macrovascular diseases. According to the pathologic investigation (Figures 1) and the results of kidney dysfunction tests (Table 1), this vitamin suppressed the development of atheromatous lesions and ameliorated kidney dysfunction by reducing serum TGF-β1, creatinine, proteinuria, and kidney weight index in the atherosclerotic rats.
The studied vitamin diminished insulin resistance in the atherosclerotic rats (Table 1) owing to the beneficial impact on insulin function. Oxidative stress and inflammation might provoke insulin resistance and vascular complications. Di-carbonyl compounds, such as MGO, are sources of oxidative stress and inflammatory markers that reduce insulin secretion, insulin resistance, and vascular complications (7, 8). The activity of the GLO system, which is the most important pathway for decreasing methylglyoxal, is disturbed in atherosclerotic rats (Table 2). According to the literature, the effect of PLP on GLO system activity in atherosclerotic patients was not studied. Previously, the protective effect of PLP against STZ-induced beta-cell dysfunction was reported (23).
The AOPPs are biomarkers of oxidative stress and inflammatory mediators and hs-CRP is a biomarker of systemic inflammation. These factors may enhance insulin resistance (24), atherosclerosis (25), and nephropathy (26). Based on Table 2, the treatment decreased the levels of AOPP and hs-CRP in normal and atherosclerotic rats (P<0.001). The elevation of antioxidant enzyme activity in surgical patients under PLP treatment (27) confirms our results regarding the antioxidant effect of PLP.
Dyslipidemia is a prevalent aspect of atherosclerosis and nephropathy (28). Treatment with PLP corrected the lipid profiles of normal and atherosclerotic rats (Table 1). Moreover, the vitamin decreased the levels of TC, LDL, and atherogenic index in diabetic rats, whereas only TG and TC were reduced in the normal group (P<0.001). As far as we know, the present investigation indicates the beneficial effect of PLP on the lipid profiles of atherosclerotic rats for the first time.
Hyperlipidemia and oxidative stress exacerbate LDL oxidation. Furthermore, hypercholesterolemia augments the changes in LDL contributing to the initiation and progression of atherosclerosis. Our results indicated that the treatment reduced the early and end oxidation products of LDL in normal and atherosclerotic rats in vitro (Tables 2 and 3). The diminishing effect of PLP on LDL oxidation in atherosclerotic rats had not been reported previously. Based on our previous studies, the treatments that inhibit the generation of the end oxidation products of LDL can prevent the development of all types of atheromatous lesions (20, 21).
Conclusion
Our findings revealed that PLP prevented the formation of atheromatous lesions and ameliorated renal dysfunction in atherosclerotic rats due to its antioxidant and anti-inflammatory properties. Furthermore, PLP had beneficial effects on insulin function, dyslipidemia, and GLO system activity. It could be concluded that PLP is useful in the treatment of diabetes.
Acknowledgements
The authors thank all those who helped them writing this article.
Ethical considerations
Ethical issues (Including plagiarism, informed consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc.) have been completely observed by the authors.
Funding and support
Ardabil University of Medical Sciences financially supported this study.
Conflicts of Interest
Authors declared no conflict of interests.
Received: 2020/06/23 | Accepted: 2020/08/12 | Published: 2020/11/11
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |