BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://journal.zums.ac.ir/article-1-6101-en.html


 , Yahya Pasdar2
, Yahya Pasdar2 

 , Behrooz Hamzeh3
, Behrooz Hamzeh3 

 , Mehdi Darabi4
, Mehdi Darabi4 

 , Mehdi Moradinazar5
, Mehdi Moradinazar5 

 , Farid Najafi *6
, Farid Najafi *6 


2- Dept. of Nutritional Sciences, School of Public Health, Kermanshah University of Medical Sciences, Kermanshah, Iran
3- Environmental Determinants of Health Research Center, Kermanshah University of Medical Sciences, Kermanshah, Iran
4- Iran University of Medical Sciences, Tehran, Iran
5- Dept. of Public Health, School of Public Health, Kermanshah University of Medical Sciences, Kermanshah, Iran
6- Environmental Determinants of Health Research Center, Kermanshah University of Medical Sciences, Kermanshah, Iran ,
✅ Female sex is a global well-established hypothyroidism-associated factor. The results of the present study suggest taking some measures to reduce hypothyroidism, by addressing the intervening factors.
CThyroid dysfunctions are generally classified as underactive and overactive thyroid gland conditions, which are known as hypothyroidism and hyperthyroidism, respectively. Nutritional intake of iodine, as the constituent element of thyroid hormones, plays an essential role in both conditions. In addition, each disease is associated with various etiologies. In areas with sufficient dietary iodine, the Hashimoto's thyroiditis (chronic autoimmune thyroiditis) is the most common cause of hypothyroidism. Graves’ is another autoimmune condition which accounts for most hyperthyroidism cases (1).
Hypo and hyperthyroidism are associated with common various factors. Hypothyroidism contributing factors are female sex (2), excess iodine (3), primary pulmonary hypertension (2), multiple sclerosis (2) and ethnicity (4). Numerous other factors may be considered as potential risk factors. Depression (5), renal disease (6), lipotoxicity (7), low seafood intake (8, 9), physical activity (10, 11) and being twins for neonates (12) have shown relation to hypothyroidism, with poorly understood underlying mechanisms.
This study was planned to investigate thyroid dysfunctions-associated factors, in a population in the West of Iran. The study was conducted to determine the correlates of hypo and hyperthyroidism in Ravansar, Kermanshah province, a hotspot for hypothyroidism. For this purpose, the baseline data were collected via Ravansar Non-Communicable Diseases (RaNCD) cohort study. With regard to the diversity of contributing factors of the two conditions, existing knowledge of the risk factors was used as a baseline throughout the study. The large initial feature space derived from our sample demanded multiple and accurate steps of feature reduction. Accordingly, standard statistical procedures were coupled with accurate machine learning method of Random Forests (RF), due to its high potential and widely-used ability to determine the feature importance (13).
Source Population and Study Settings
Baseline data were collected from recruitment phase of RaNCD cohort study, in the West of Iran in 2018. The RaNCD has been started as a part of Prospective Epidemiological Research Studies in Iran (PERSIAN) in 2014, to determine the burden of Non-Communicable Diseases (NCD). The study was approved by the Ethics Committee of Kermanshah University of Medical Sciences (ethics approval number: KUMS.REC.1394.318). We recruited ~10,000 adults in the age range of 35-65 years, from both rural and urban areas of Ravansar city. Data were analyzed to understand the relationship between the development status, thyroid condition and an input set of diverse features. For more details about data collection, variables grouping and literature-guided categorizations, see the Supplementary Materials.
Machine learning-based dimensionality reduction and conventional statistical analysis
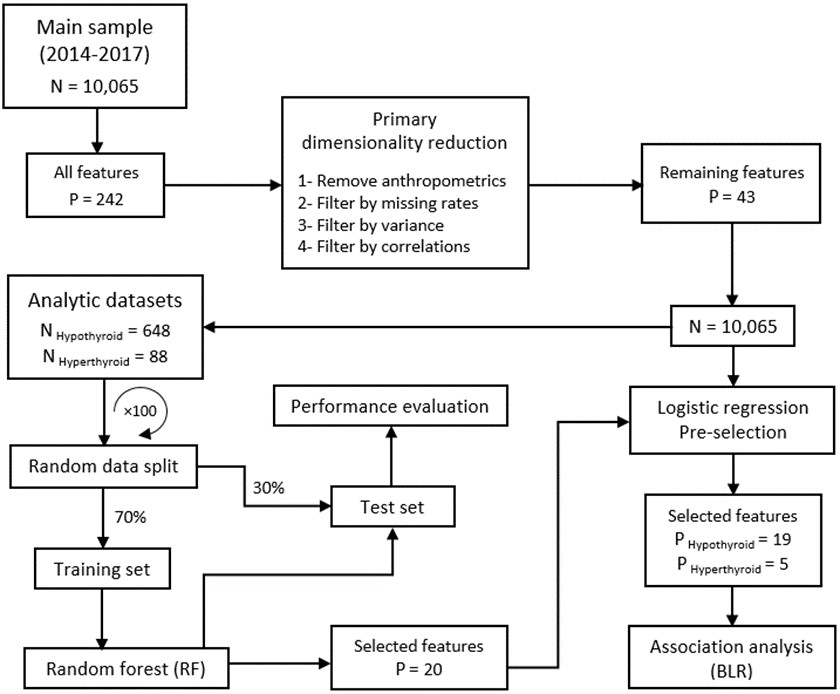
Dimensionality reduction of data and effect size modeling steps are shown in Figure 1. For details of the feature selection steps, including RF implementation, see the Supplementary Material. Chi-square test was used for analyzing the categorical variables. P-value<0.05 was deemed statistically significant, unless otherwise were specified. All preprocessing, model building and effect size estimation steps were performed in the R statistical environment by the following packages including RF (14), DMwR (15), pROC (16) and mice (17).
Descriptive Characteristics of Participants
Baseline demographic profiles of euthyroid, hypothyroid and hyperthyroid samples of the study population have been compared in Table 1. This cohort of adults comprises 4752 (47.4%) male and 5269 (52.6%) female. The average age was 48.1 ± 8.2 years. The prevalence of hypo- and hyperthyroidism conditions were 3.21% and 0.43 %, respectively.
Thyroid Dysfunction-Associated Features
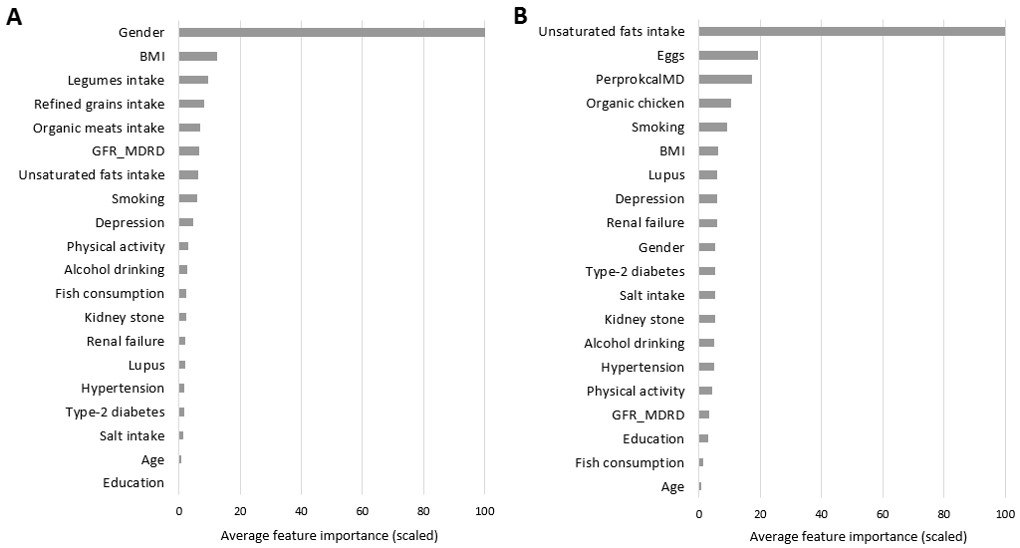
The identified variables by the RF model, which were considered as important features are shown in Figure 2. Hypothyroidism-related variables are listed in Table 2. Female sex was known as a powerful risk factor for hypothyroidism; the odds ratio of the disease was 524% higher for women. The middle-aged subjects were 56% more likely to be categorized in hypothyroidism conditions compared to the younger individuals.
Consuming more than 10 gr salt per day increased the chance of the disease by a factor of 1.87. Kidney stone also demonstrated a significant association with underactive thyroid gland disease with an odds ratio of 1.35. Hypothyroidism chance was 107% higher for depressed participants compared to non-depressed ones. In a significant borderline association, 1 gr increased daily intake of unsaturated fats was linked to 1% higher odds of hypothyroidism.
None of the six final selected features demonstrated significant association with hyperthyroidism conditions.
Table 1. Potential associations of thyroid dysfunction profile, with demographic, life-style and medical characteristics, in the study sample
| Euthyroidism | Hypothyroidism | Hyperthyroidism | P-value | |
|---|---|---|---|---|
| Total, N (%) | 9697 (96.34) | 324 (3.21) | 44 (0.43) | |
| Age, N (%) 35-45 years 45-55 years 55-65 years |
4284 (96.74) 3200 (95.58) 2213 (96.67) |
124 (2.8) 132 (3.94) 68 (2.97) |
20 (0.45) 16 (0.47) 8 (0.35) |
0.057 |
| Sex, N (%) Male Female |
4684 (98.75) 4950 (94.12) |
42 (0.88) 282 (5.36) |
17 (0.35) 27 (0.51) |
<0.001 |
| BMI (kg/m2), N (%) <25 25-29.9 30-34.9 ≥35 |
2884 (96.94) 4203 (96.59) 1994 (95.5) 533 (93.84) |
75 (2.52) 129 (2.96) 86 (4.12) 34 (5.98) |
16 (0.54) 19 (0.43) 8 (0.38) 1 (0.17) |
<0.001 |
| Education (years), N (%) Illiterate 1-5 6-9 10-12 ≥13 |
2395 (96.1) 3680 (95.73) 1623 (96.89) 1232 (97.0) 767 (97.83) |
87 (3.49) 151 (3.92) 43 (2.56) 30 (2.36) 13 (1.65) |
10 (0.40) 13 (0.33) 9 (0.53) 8 (0.63) 4 (0.51) |
0.008 |
| Smoking, N (%) No Former Current |
7695 (95.86) 809 (97.58) 1164 (98.72) |
295 (3.67) 15 (1.8) 13 (1.1) |
37 (0.46) 5 (0.6) 2 (0.17) |
<0.001 |
| Alcohol consumer, N (%) No Yes |
9070 (96.18) 627 (98.74) |
320 (3.39) 4 (0.62) |
40 (0.42) 4 (0.62) |
<0.001 |
| Physical activity, N (%) Low Moderate High |
6 (85.71) 166 (97.64) 9523 (96.32) |
1 (14.28) 3 (1.76) 320 (3.23) |
0 (0.0) 1 (0.6) 43 (0.43) |
0.403 |
| Salt taste, N (%) Less salty Average Salty |
8412 (96.47) 782 (95.71) 471 (94.95) |
273 (3.13) 28 (3.42) 22 (4.43) |
34 (0.39) 7 (0.85) 3 (0.60) |
0.147 |
| Fish intake, N (%) Never Occasional Frequent |
1016 (95.93) 3780 (95.74) 4873 (96.87) |
38 (3.58) 148 (3.74) 138 (2.74) |
5 (0.47) 20 (0.50) 19 (0.37) |
0.072 |
| GFR_MDRD1, N (%) < 60 ml/min per 1.73 m2 ≥ 60 ml/min per 1.73 m2 |
1037 (94.44) 8597 (96.55) |
54 (4.9) 270 (3.03) |
7 (0.63) 37 (0.41) |
0.002 |
| Type-2 diabetes, N (%) Not developed Developed |
8848 (96.38) 783 (95.6) |
293 (3.19) 31 (3.78) |
39 (0.42) 5 (0.61) |
0.483 |
| Hypertension, N (%) Not developed Developed |
8174 (96.56) 1499 (95.17) |
255 (3.01) 68 (4.31) |
36 (0.42) 8 (0.50) |
0.023 |
| Depression, N (%) Not developed Developed |
9387 (96.51) 298 (91.13) |
296 (3.04) 28 (8.56) |
43 (0.44) 1 (0.30) |
<0.001 |
1 Glomerular filtration rate obtained from Modification of Diet in Renal Disease formula.
Table 2. Variables with significant association with hypothyroidism
| Predictor | OR (95% CI) | P-value |
| Age (years) 35-45 45-55 55-65 |
1 1.56 (1.18-2.07) 1.17 (0.80-1.72) |
0.002 0.403 |
| Sex Male Female |
1 6.24 (4.13-9.63) |
<0.001 |
| Salt intake (gr/day) <6 6-10 >10 |
1 1.15 (0.74-1.70) 1.87 (1.15-2.90) |
0.505 0.007 |
| Unsaturated fats intake (gr/day) | 1.01 (1.00-1.02) | 0.025 |
| Self-reported physician-diagnosed kidney stone No Yes |
1 1.35 (1.02-1.78) |
0.031 |
| Self-reported physician-diagnosed depression No Yes |
1 2.07 (1.34-3.09) |
<0.001 |
| Abbreviations: OR, Odds ratio; CI, Confidence interval. | ||
Figure 1. Schematic flow for dimensionality reduction and association analysis.
Figure 2. Feature importance based on mean decrease in prediction accuracy, obtained from 100 Random Forest model implementations, and scaled to a maximum of 100, for (A) Hypothyroidism, and (B) Hyperthyroidism.
Discussion
This study was performed to determine the possible risk factors of hypothyroidism and hyperthyroidism in the population from the West of Iran. Preliminary analysis of descriptive features demonstrated that Ravansar is an endemic area for hypothyroidism condition. Comparing to the recent reports, global prevalence of hyperthyroidism is 0.2-1.3 % (1), which indicates a normal prevalence of disease in the Ravansar cohort. On the other hand, global epidemiological data of hypothyroidism showed a prevalence of 1-2 % (1), thus the region is a hotspot for hypothyroidism.
Based on the results, female sex has a remarkable contribution to hypothyroidism, which is considered as one of the most established risk factors (2). Depression was found as another highly related factor to hypothyroidism. Despite abundant literatures, the connection between hypothyroidism and depression was not clearly defined (18). Depression is known as a neurological manifestation of hypothyroidism. On the other hand, depressive patients have demonstrated a higher frequency of hypothyroidism (5).
Hypothyroidism has been shown to be correlated with age; it is more common among people over the age of 60 (19). In our study, the younger age range of 45-55 years demonstrated a significant association with the disease, while no association was found for older age group of 55-65 years. Besides other factors, ethnic and regional environmental differences may explain such observations (20). Overall, thyroid dysfunction rate was higher in a Japanese study with the mean age of 51.3 ± 9.0 (21), compared to another study which was done on Brazilians of Japanese descent with the older age range of 56.9 ± 12.5 (22).
Salt consumption was associated with hypothyroidism in Ravansar area. Though the iodine intake did not have a specific representative in the study features, iodine deficiency may not be the cause of hypothyroidism prevalence in the region, as Iran is known as an iodine-replete country after the nationwide adoption of iodized salt, in 1996 (23). In contrast, the remarkable positive correlation between salt consumption and hypothyroidism indicates that the excess iodine delivery through salt usage may be the cause for the thyroid dysfunction in Ravansar population; this condition is known as iodine-induced hypothyroidism (3, 24).
Regardless of zero, a significant borderline association was detected between ingestion of unsaturated fats and hypothyroidism. In agreement with this finding, lipotoxicity was introduced as a potential risk factor for the prevalence of subclinical hypothyroidism, recently (7).
We found that kidney stone was associated with hypothyroidism. There are various interplay mechanisms between kidney and thyroid function in both normal and disease conditions of the organs (6). No direct connection has been reported between nephrolithiasis and thyroid diseases. Cumulative kidney stone size is known as an independent predictor of chronic kidney disease (CKD) in kidney stone formers with a cumulative stone size up to 20 mm (25). Thus, underactive thyroid may result from progressive damage to kidneys by kidney stones.
In the present study, hyperthyroidism w:as char:acterized by anti-thyroid drugs intake and iodine radioisotope therapy. None of the existing variables showed significant relation with hyperthyroidism. While cited negative result limits the conclusion about the actual contributors to the hyperthyroidism, the endemic hypothyroidism, its specific medication (Levothyroxine) or its associated risk factors (specifically the high salt intake) may provide bases to explain hyperthyroid cases. Levothyroxine treatment accounts for up to 50% of cases of subclinical hyperthyroidism. It is particularly for the low threshold for the treatment initiation (26). In addition, excessive iodine load provides a rich substrate to increase thyroid hormones production in some susceptible patients. It leads to a transient or permanent condition which is called iodine-induced hyperthyroidism (the Jod-Basedow phenomenon) (3). Based on our results, the high iodine intake through too much consumption of iodized salt is likely to contribute to hyperthyroidism occurrences in Ravansar samples (4).
Apart from restriction characteristics of the cross-sectional design, one limitation of the present study was disease classification regardless of the subtypes (overt, subclinical and central types). The second limitation was that our feature space did not cover all the potential risk factors. In addition, specific age range of the study population may limit generalizability of the results, though it renders the findings specifically. To compensate the limitations, longitudinal studies should be performed; they could investigate the causality between thyroid dysfunction subtypes and a largest set of potential determinants.
Conclusion
In conclusion, the results of the present study provided implications for facing up to the high prevalence of hypothyroid cases in the study region. Female sex is a global well-established risk factor for hypothyroidism, whereas the other predictors may be population-specific, and may be addressed as the interfering factors. Thus, policies which lead to modify salt intake, kidney stone formation prevention and depression reduction, will lessen the hypothyroid prevalence in this region. Our study also highlighted the promising utility of machine learning tools in combination with conventional statistical procedures, to filter out the unrelated variables from large sets of initial potentially associated features.
Acknowledgements
RaNCD is a part of PERSIAN national cohort, and we would like to thank Professor Reza Malekzadeh, Deputy of Research and Technology at the Ministry of Health and Medical Education of Iran and Director of the PERSIAN cohort. We thank Dr. Hossein Poustchi, Executive Director of PERSIAN cohort, for all their supports during designing and running of RaNCD. No potential conflicts of interest was reported to this article. This study was supported by Ministry of Health and Medical Education of Iran, and Kermanshah University of Medical Sciences (Grant No: 92472).
Funding and support
This research is associated to the project number 980785 of Kermanshah University of Medical Sciences.
Conflicts of Interest
Authors declared no conflict of interests.
Received: 2020/07/3 | Accepted: 2020/12/20 | Published: 2020/12/30
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |