BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://journal.zums.ac.ir/article-1-6281-en.html


 , Mohammad Kazem Sharifi Yazdi2
, Mohammad Kazem Sharifi Yazdi2 

 , Zahra Rajabi3
, Zahra Rajabi3 

 , Farzaneh Amin Harati1
, Farzaneh Amin Harati1 

 , Farhad Nikkhahi1
, Farhad Nikkhahi1 

 , Sara Sharifi Yazdi4
, Sara Sharifi Yazdi4 

 , Gholamreza Hassanpour5
, Gholamreza Hassanpour5 

 , Alireza Monadi Sefidan6
, Alireza Monadi Sefidan6 

 , Mohammad Mehdi Soltan Dallal *7
, Mohammad Mehdi Soltan Dallal *7 


2- Zoonosis Research Center, Tehran University of Medical Sciences, Tehran, Iran
3- Food Microbiology Research Center, Tehran University of Medical Sciences, Tehran, Iran
4- Medical Student, Faculty of Medicine, Tehran University of Medical Sciences, Tehran, Iran
5- Center for Research of Endemic Parasites of Iran, Tehran University of Medical Sciences, Tehran, Iran
6- Dept. of Medical Laboratory Sciences, Faculty of Allied Medical Sciences, Tehran University of Medical Sciences, Tehran, Iran
7- Dept. of Pathobiology, Faculty of Public Health, Tehran University of Medical Sciences, Tehran, Iran ,
✅ Additional studies are needed for better understanding of the interaction between phage, microorganisms and human host before applying phage therapy on a large scale.
Salmonella infections are spread primarily by conta-minated foods, poultry, eggs, and milk. An infection in humans can occur after drinking contaminated water or ingesting uncooked contaminated eggs, milk, and meat originating from poultry, cattle, or swine.Thus Salmonella infections represent a major concern to public health, animals, and food industry worldwide. Human health can be harmed as a result of direct contact with infected animals, blood, urine, and feces. Antibiotics have been used more often to suppress bacteria in animals and increase food production, hastening the proliferation of antimicrobial-resistant bacteria (1). When animals are kept on the farm the methods of transmission are usually more complex, and Salmonella strains and serotypes can remain on farms simultaneously for long periods (2). The primary method for identification is serotyping, with over 2400 serovars identified. Methods for discrimination within serovars of clinical and epidemiological import-ance include phenotypic tests such as phage typing. Divisions at different levels within serotypes are important in epidemiological studies and at the local level in monitoring these pathogens (3). Antibiotic use has been used to control bacteria has accelerated the emergence of antimicrobial-resistant bacteria .The rapid emergence of resistant bacteria is occurring worldwide (4). Foodborne outbreaks due to Salmonella still pose a prominent risk to public health. As chicken meat is a good reservoir for Salmonella, it is important for chicken processing plants to continuously optimize methods to reduce the incidence of Salmonella on their products .Therefore, monitoring the presence and prevalence of Salmonella in poultry flocks is the first step to control this zoonosis, which will continue in the later stages of the poultry chain with microbiological control of poultry meat, eggs, and their products (5). Antimicrobial resistance is recognized as one of the greatest threats to human health worldwide. The resistant infections in various tissues and organs that cannot be treated with antibiotics are considered as important healthcare problems that can endanger human life. The antibiotic resistance crisis has been attributed to the overuse and misuse of these medications, as well as a lack of new drug development by the pharmaceutical industry due to reduced economic incentives and challenging regulatory requirements (6). The practice of phage therapy, which uses bacterial viruses (phages) to treat bacterial infections, has been around for almost a century by Felix d’Herelle (7). The lethal effect of bacteriophages on their bacterial hosts has been known since their discovery (8). Today, phage therapy can be considered as an alternative treatment option that does not any major side effects on eukaryotic cells (9). Further-more, with more detailed studies, the limitations of phage therapy, have been solved and have made them good options for use in treatment systems (10,11). Therefore, the present study aimed to isolate an effective titer of phages against Salmonella infantis and determine the phage characteristics. Further, the specificity of bacteri-ophage for Salmonella infantis isolates was determined in vitro.
Bacterial preparation and susceptibility testing
Laboratory studies of this study included bacteriophage sampling and isolation in Food Microbiology Laboratory of School of Public Health, Tehran University of Medical Sciences. Salmonella infantis (ATCC 51741) serotypes were obtained from Faculty of Veterinary Medicine, University of Tehran. Antibiotic susceptibility was tested using E-test (AB.BioDisk, Solna, Sweden) in accordance with the guidelines from the Clinical and Laboratory Standards Institute (CLSI.2018).
E.test method for determination of susceptibility of Salmonella infantis to ciprofloxacin antibiotic
The E-test (AB.Biodisk, Solna, Sweden) was used .In this method, after preparation of a bacterial suspension by a semi-McFarland method, it was transferred onto a Mueller Hinton Agar plate by a sterile swab using a cotton seed dressing method, and then the E.test strips, which represented the ciprofloxacin antibiotic, were applied to the agar plate And after 24 hours of incubation at 37 °C, the area of the inhibition zone was triangular, and then by referring to the table provided by the company E.test tape (AB.BioDisk, Solna Sweden) sensitivity Serotype Infec-tion of Salmonella was determined by the antibiotic.
Bacteriophage isolation and purification
The bird stool sample was first mixed in a certain amount of distilled water. Using centrifuge (Sigma, Germany), the mixed solution was centrifuged at 15 00 gr for 20 minutes at 4 ° C. The centrifuge solution was then filtered with a 0.22 μm filter (JetBiofil, China). In order to search the plaque, a double-layer cultures were used on an agar plate as follows. A portion of the centrifugation solution and a few milliliters of the 12-hour culture of Salmonella bacterial enterotoxin were added to the Brain Heart Infusion (BHI) medium (Merck, Germany) with 0.7% agar and placed on a plate containing a BHI fluid with a base of 5 / 1% agar was added superficially (12). After closing the agar in the medium and incubating at 37 ° C for 24 hours plaques were observed (transparent points caused by bacterial lysis of bacteria by bacteri-ophage). The plaques were removed by sterile Pasteur pipette and added to the BHI medium for 24 hours for enrichment, then the enriched phage was used to the research of plaques. In order to purify the phage, all phases were repeated five times (13).
Morphological assessment of the phage
The isolated bacteriophage was examined for size and morphology using a transmission electron microscope (TEM). For this purpose, a drop of phage was poured over a carbon-coated copper grade and was then painted with 2% uranyl acetate.
Bacteriophage titration
Bacteriophage titration was carried out in three steps. First, Salmonella infantis was inoculated on LB agar and plated at 37 ֯C for 24h. Then, a single colony of the bacterium was inoculated in 25ccs of tryptone broth at 37 ֯C. Finally, 1cc of SM buffer was pipetted in a tube and a dilution of 10-10 was prepared using serial dilution. Besides, 300μL of bacterial culture was poured in 9 tubes, and 100μL of each dilution was serially added to each tube. Tubes were then vortexed and place at room temperature for 20min.
Nine tubes were labeled and 4ccs of molten agar (40 ֯C-50 ֯C) were poured in tube No. 1 and the mixture in the tube was added to agar using a sterile pipette. After vortex, this suspension was transferred to Luria–Bertani agar (LB). This procedure was carried out for each dilution and the plates were incubated at 37 ֯C. Finally, a plaque assay was carried out to determine plaque formation.
Phage susceptibility to heat
A certain concentration (aliquot) of phage suspension (1×108 CFU/ml) was inoculated at 4, 25, 37, 50, 60, and 70 ֯C at pH=7 for 60 min. Bacteriophage titration was carried out using the Double-Layer Agar method.
Phage susceptibility to pH
A certain concentration (aliquot) of phage suspension (1×108 CFU/ml) was inoculated at 4 ֯C at a pH range of 3 to 12 for 60 min. Bacteriophage titration was carried out using Double-Layer Agar.
Determining the Host and Specificity of Bacteriophage
Spot test was used to determine the bacteriophage specificity of Salmonella infantis. During this process, two groups were selected for the specificity of the bacteriophage:
Group 1: Non-Salmonella bacteria (Staphylococcus aureus (ATCC 43300), Shigella dysenteriae (ATCC 13313) and Entero-pathogenic Escherichia coli (ATCC 43887)
Group 2: Salmonella infantis serotypes
All the bacteria were placed in BHI Broth for 24 hours. 100 μl of the culture suspension was mixed with ml of agar-BHI medium and repeated with BHI agar for making two-layer Pure Plate. When medium became solid 10 μl of the bacteriophage suspension was added and incubated at 37 ° for 24 hours. Bacteriophage sensitivity was determined by translucent area of non-growth.
Bacterial invasion and the effect of a specific bacteriophage using HEp-2 cell line
MEM medium containing 10% FBS is used to culture HEp-2 cells. Thus, for every 90 ml of MEM, 10 ml of deactivated FCS is used. Then add 2 ml of 10% sodium bicarbonate and 1ml of penicillin-streptomycin solution and 2ml of L_glutamine and thus the environment is ready for consumption. But we have to make sure it is sterile before use. If necessary, 5% and 2% FBS media can be prepared in the same way, which is mostly used to maintain or replace the cell culture medium. At this point, the 1.5 ml cell suspension was added to sterile Leighton tubes to cover the entire lamellar surface of the tube. Then tubes were placed horizontally in an incubator at 37 ° C. After 24 hours, the medium was replaced with 1.5 ml of warm minimum essential medium (MEM). The previous culture was discarded and the warm antibiotic-free MEM medium was added one day before the experiment respectively. The tubes were placed again horizontally in an incubator at 37 ° C for testing the next day. To infect the cell line, at first, the culture medium of HEp-2 cells in the previously prepared Leighton tubes was discarded and cells wash thoroughly with 1.5 ml of Earle's Salt. Then tubs were placed horizontally in an incubator at 37 °C for 3 hours. During 3 hours, the invading bacteria use their invasive power to infect the cells, and after 3 hours, the cell culture medium, which has turned yellow due to the growth of bacteria, is discarded and the cells were treated several times with warm Salt and paint finally.
Addition of bacteriophage to infectious HEp-2 cell line
A definite dilution of an overnight culture of Salmonella infantis was added to LB broth and vortexed at 150 rpm at 37 ֯C until reaching the optic density of 0.6 at 650 nm. Next, a certain concentration of bacteriophage (1×108 CFU/ml) was prepared. Isolated bacteriophage was inoculated on the HEp-2 cell line and was then incubated at 37 ֯C for 24 h. Cells were then assessed under microscopy after 24 h.
Antibiotic susceptibility testing
The Standard strain of Salmonella infantis was confirmed by E-test according to CLSI(2018) guideline (Fig. 1).
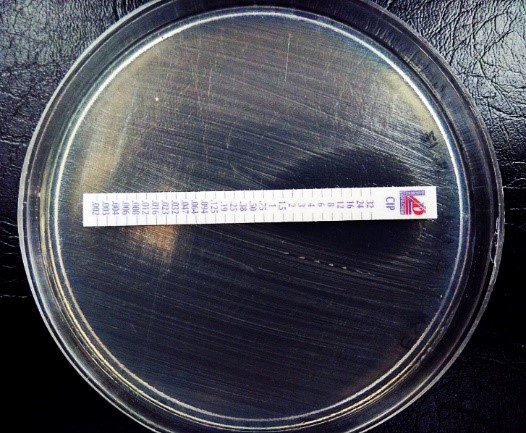
Figure 1. Bacterial susceptibility to Ciprofloxacin
Determination of bacteriophage morphology
Using microscopy, bacteriophages with an icosahedral head of approximately 90 nm were observed along with a very short tail 135 nm. Transmission electron microscopy showed isolated bacteriophages belonging to the Myoviridae family (Fig. 2).
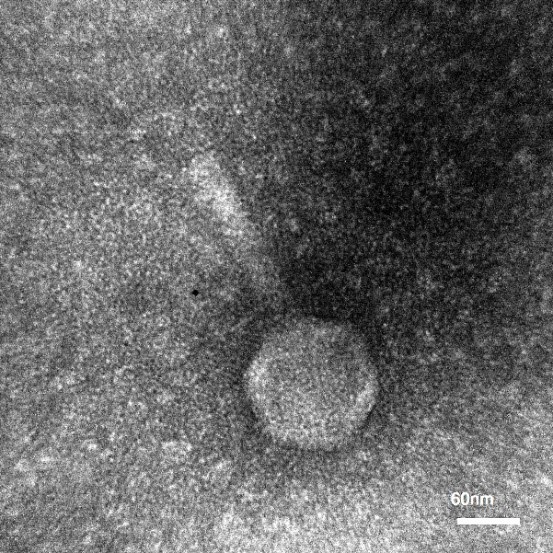
Figure 2. Determination of morphology of Myovirida family
Bacteriophage titration
To count the bacteriophage in the sample, the serial dilution method was used and double-layer agar was performed for plaque assay. The results of the double-layer agar assay showed that the titer of bacteriophages was 1.8×107 PFU/mL. To be able to report the number of plaques, 30-300 plaques should be formed on the media (Table1). Bacterial dilution of 104 was used to report the number of phages according to the following formula (14):
Number of phage in 1ml suspention=1.8×107 PFU/mL..
Table 1. No. of bacteriophage plaques in each sample
| No. of plaques in different dilutions of bacteriophage suspensions | |||
| 106 | 105 | 104 | Dilution of specific bacteriophage |
| 52 | 98 | 180 | Salmonella infantis |
Susceptibility of bacteriophage to heat
As indicated in Table 2, this bacteriophage was stable at 4 ֯C, indicating the shelf life of 3 months in the refrigerator (Table 2).
Table 2. Results of bacteriophage quantification after exposure to different ranges of heat.
| 80 | 70 | 60 | 50 | 37 | 25 | 4 | Temperature (֯C) |
| 0 | 0 | 104×1 | 105×1 | 108×1 | 108×1 | 109×1 | No. of phages (CFU/ml) |
Susceptibility of bacteriophage to pH
Bacteriophages were quantified at different pH values. Each quantification was carried out in triplicates (Table3).
Table 3. Results of the quantification of isolated bacteriophage after exposure to different pH values.
| 10 | 9 | 8 | 7 | 6 | 5 | 4 | 3 | pH |
| 106×1 | 107×1 | 1010×1 | 109×1 | 108×1 | 107×1 | 106×1 | 105×1 | No. of phages ( (CFU/ml |
Bacteriophage specificity
The specificity of bacteriophage against Salmonella infantis isolate was confirmed using the Spot test (Table4). Also, Salmonella infantis Susceptibility to bacteriophage was observed based on the formation of inhibition zone.
Table 4. The Effect of Isolated Bacteriophage on a Variety of Gram-Positive and Negative Bacteria
| Bacteria Lysis |
| Salmonella enterica serovar Infantis (ATCC 51741) + Staphylococcus aureus (ATCC 43300) _ Shigella dysenteriae (ATCC 13313) _ Enteropathogenic E.coli (EPEC) (ATCC 43887) _ |
The effect of isolated bacteriophage on Salmonella infantis using HEP-2 cell-line
Isolated bacteriophages under sterile conditions were applied to pre-prepared infectious cell lines and a tube with healthy cells was applied for control. Then they were incubated for 24 hours at 37 °C. The next day, we removed, stained, and examined them under a microscope (Figure.3). According to the images related to the study of the effect of isolated bacteriophage on the HEp-2 cell line infected with Salmonella infantis, the isolated bacteriophages showed a good ability to reduce the number and eliminate the studied bacteria. The bactericidal effects of isolated bacteriophage against Salmonella infantis were evaluated as completely positive. As shown in the figure, infected HEp-2 cell lines have lost their original state (deformation) and intercellular connections have been largely lost. After adding the specific bacteriophage of this infectious bacterium, after the incubation period as expected, it was observed that the bacteriophages were able to lysis the cells of their specific bacterium and largely removed this infectious bacterium, which As a result, they maintain the original shape and condition of the cells and the stability of intracellular com-ponents, which is the result of the absence of bacterial contamination.
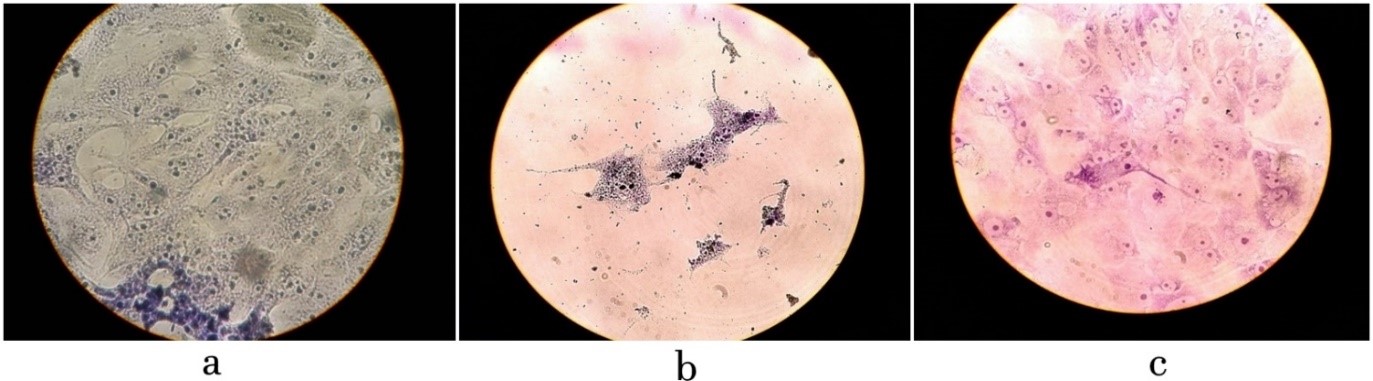
Figure 3. a. intact HEp-2 cell line after the incubation period and staining b. disruption of intercellular junctions and infection of HEp-2 cell line by Salmonella infantis c. Effect of specific bacteriophage on HEp-2 cell line infected with Salmonella infantis
Discussion
Antibiotics such as third-generation fluoroquin-olones and cephalosporins are used to treat serious S. enterica infections due to the high prevalence of antibiotic resistance in S. enterica isolates. As a result, other antimicrobials must be used to supplement antibiotics.Phage therapy was recognized as alternative method to threats and prevent antibacterial resistance (9).Clinical manifestations of human salmonellosis are from subclinical inflammation to bacteremia (severe bacteria), meningitis (inflammation of the membrane around the brain and the spinal cord) and other forms of extra-intestinal infections (15). There are more than 2300 known serotypes of Salmonella enterica (16), which show great differences in pathogenicity (17). In this regard, the need to replace antibiotics with antimicrobials such as bacteriophage with is necessary (18). The isolated bacteriophages were evaluated to determine the host and susceptibility of the contamin-ating bacteria. Salmonella infantis serotype was speci-fically susceptible to this bacteriophage. The trans-parency of the inhibition zone in the Spot test was well seen in this serotype. The positive effect of separation bacteriophage in controlling and eliminating the pathogen was proved by Timothy K Lu (19). In this study at different conditions and tests, including the reduction of opacity in a tube containing serotypes, the creation of plaque on a bacterial cell-based serotypic agar plate and the Spot test, proved the ability of lysis and ultimately the elimination of bacterial pathogens. The results of this study are consistent with other studies that suggested the use of bacteriophage as a therapeutic strategy for reducing bacterial populations and controlling food contamination (20,21). The other bacteria such as Staphylococcus aureus, Entero-pathogenic Escherichia coli, Shigella flexneri and Yersinia enterocolitica were studied. They did not show sensitivity to these isolated bacteriophages (22-25). These results indicated that this agent was completely specific. Our results are in agreement with another researcher that found phages are more appropriate and more beneficial than antibiotics and are more specific than antimicrobial agents (26, 27). The results of this study revealed the specificity of isolated bacteriophage in comparison to serotype of Salmonella. Hence, isolated bacteriophages can be identified as an indicator of the contamination associated with Salmonella serotype infection, which is consistent with studies by Amit Singh et al. (28). The present study aims to evaluate the antibacterial activates of isolated lytic bacteriophage against ciprofloxacin-resistant strain of Salmonella infanits in vitro conditions. In this study antibacterial activates of isolated lytic bacteriophage against ciprofloxacin-resistant strain of Salmonella infantis in vitro condition was evaluated.Our findings showed that the isolated bacteriophaged had a specific inhibitory of Salmonella infantis. The sensitivity of Salmonella s immunization to its specific bacteriophage was well demonstrated. The sensitivity of the region or zone of inhibition was observed. In other intestinal pathogenic bacteria, this sensitivity was not observed. In the study of Rattanachaikunsopon and others, Salmonella bacteri-ophage was isolated from Salmonella typhi alone, and other Salmonella bacteria were not susceptible to this phage, which is consistent with the results of this study (29).In this study, isolated bacteriophage was evaluated for controlling and eliminating the bacterial agent of Salmonella infantis serotype bacteria. Results in different conditions and tests, including the reduction of opacity in a tube containing serotypes, the creation of plaque on agar-based plate containing bacterial serotype and the Spot test, proved that this therapeutic agent, the ability of lysis and in Ultimately, the bacterial pathogens have been eliminated, which is consistent with the study of Nikkhahi et al. (2017), which was performed on the evaluation of the efficacy of isolated bacteriophages in salmonellosis-induced Salmonella enteritidis mice (30).In this study, isolated bacteriophage was evaluated to determine the specific host and sensitivity of various bacteria to it. Serotype Infection of Salmonella bacteria to a specific bacteriophage itself showed a complete sensitivity to the results of the Spot test and the other pathogens studied did not show a sensitivity to this isolated bacteriophage (31,32). These results indicated the specificity of this therapeutic agent. That is consistent with investigated the isolation of E. coli bacteriophage from raw sewage and determining its specific host (14).
Conclusion
Phage therapy could be used as an alternative method for the treatment of bacterial infection.Our study has shown that bacteriophage or a phage combination can reduce or completely eliminate the intestinal bacterium Salmonella enterica serotype infantis in In Vitro condition.
Acknowledgements
This article is the result of a plan approved by the Food Microbiology Research Center of Tehran University of Medical Sciences and Health Services under the contract number 32411 and approved by the Committee for Research Ethics (no.IR.TUMS.VCR.-REC.1395.773). We are grateful to the Vice-Chancellor of Research in Tehran University of Medical Sciences who sponsor this research project.
Conflicts of Interest
The authors declare that they have no conflict of interest.
Received: 2020/11/1 | Accepted: 2021/03/14 | Published: 2022/01/31
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |



