BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://journal.zums.ac.ir/article-1-7350-en.html
2- School of Medicine, Islamic Azad university of Medical Science, Tehran, Iran
3- Department of Radiology, School of Medicine, Zanjan University of Medical Sciences, Zanjan, Iran
4- Department of Genetics and Molecular Medicine, School of Medicine, Zanjan University of Medical Sciences, Zanjan, Iran & Metabolic Diseases Research Center, Zanjan University of Medical Sciences, Zanjan, Iran ,
Breast cancer (BrCa) is the most broadly known malignancy among women and the world’s leading reason for cancer mortality (1, 2). Each year, approximately 1.5 million women (25% of entire malignancy cases) are identified with BrCa globally (3). In 2017, over 250,000 novel cases of BrCa were described in the USA, with 12% of all women in the country expected to be diagnosed with the disease at some point in their lives (4). Achieving improved outcomes for BrCa through early detection, diagnosis, and management is challenging in developing nations due to resource and infrastructure limitations (5). In developed countries like the USA, it was assessed that 232,340 women will be detected with BrCa in 2013, with 39,620 deaths from the disease (2).
Guanylate-binding proteins (GBPs) were first recognized in human fibroblasts as proteins activated by IFN-γ. These proteins are related to dynamin and belong to the large GTPase superfamily, sharing similar structural and biological features (6, 7). GBP1 and GBP2 are the greatest induced members of the human GBP group in cells or tissues exposed to IFN-γ (8-10). GBP1 can modulate the inhibitory effects of inflammatory cytokines on endothelial cell proliferation, migration, and invasion, as well as epithelial tumor cell behavior (10-14). Furthermore, GBP1 expression in colorectal malignancy has been linked with decreased cancer aggressiveness and enhanced development (14, 15).
Conversely, GBP2, a member of the p65 GBP family of interferon-gamma-inducible GTPases, plays a role in the innate immune reaction to microbial infections and has been known as a regulator of tumor cell proliferation and dissemination (16-18). GBP2 was recently identified as a TGF-β target gene activated in metastatic BrCa cells (19). GBP2 expression has also been associated with improved prognosis in BrCa patients and may play a role in T-cell defense against the disease. (19). In NIH 3T3 fibroblasts, GBP2 expression suppressed Rac activation and matrix metalloproteinase-9 production, which recommend a role in regulating cancer metastasis. However, the pathways and molecular processes by which GBP2 regulates cancer metastasis are largely unexplored (20). GBP2 may have a role in tumor formation, according to recent findings (20-22). By preventing NF-Kappa B and Rac protein, GBP2 blocks the induction of matrix metalloproteinase-9 (MMP-9) (22, 23).
The potential function of GBP family members in BrCa immune responses is poorly understood. Therefore, we primarily studied GBP prognostic and expression value in BrCa by mining the Kaplan-Meier plotter, UALCAN, and TIMER databases. Using TIMER databases, we also investigated the relation between GBP expression and immune infiltrating plenty.
2.1 UALCAN
UALCAN was utilized to analyze the seven GBP family members’ transcriptional expression in BrCa tissues and adjacent normal tissues. T-tests with unequal variance was used to assess the meaning of differences in transcriptional stages. (p < 0.05 considered statistically remarkable.)
2.2 bc-GenExMiner v4.2
The bc-GenExMiner v4.2 was used to assess the association between GBP expression and clinicopathologic parameters of BrCa, such as age, progesterone receptor (PR), estrogen receptor (ER), nodal status, Scarff-Bloom-Richardson (SBR) grade, and human epidermal growth factor 2 (HER2), molecular subtype. Dunnett-Tukey-Kramer and Welch's tests were utilized to assess the differential mRNA expression of GBPs in BrCa cases with varied clinical and molecular characteristics, with p < 0.05 considered statistically remarkable.
2.3 TCGA Database
The Cancer Genome Atlas (TCGA) is a breakthrough cancer genomics database that molecularly considered over twenty thousand primary tumors and matched usual samples spanning thirty-three malignancy forms. TCGA conducts systematic studies to recognize the mechanisms of cancer cell incidence and progression, to find novel diagnosis and therapy options.
2.4 Human Protein Atlas
This database is a unique website leading the effort to map entirely human proteins in cells, tissues, and organs through systems biology in the human body, mass spectrometry-founded proteomics, antibody-based imaging, and transcriptomics. In current study, the direct comparison of protein expression of various GBP family participants between BrCa tissues and normal tissues was done using immunohistochemistry images.
2.5 GeneMANIA
The genetic and protein relationships, co-expression, pathways, co-localization, and protein domain relationship of the seven GBP family memberships and their ligands were investigated using GeneMANIA (http://www.genemania.org).
2.6 STRING
STRING is a database that assembles, scores, and integrates all publicly existing protein-protein interaction data sources and provides computational predictions of probable activities. This network analysis of the seven GBP family participants and their ligands was performed.
2.7 TIMER
TIMER was used to confirm the comparative expression of GBPs between BrCa and normal samples by the Diff Exp module and to investigate the relationship between GBP expression and immune infiltrating cells (CD4+ T cells, B cells, macrophages CD8+ T cells, neutrophils, and dendritic cells) with the Gene module and with immune indication sets of tumor-infiltrating immune cells. The Spearman coefficient was used to express the relationship, and purity was used to adjust it. The Wilcoxon test was utilized to evaluate differential expression.
2.8 Kaplan-Meier Plotter
This database was utilized to assess the prognostic value of different GBP mRNA expressions in BrCa patients. Overall survival (OS), post-progression survival (PPS), relapse-free survival (RFS), and distant metastasis-free survival (DMFS) were analyzed for all BrCa patients. Patients were then divided into subgroups based on their molecular subtype. The cutoff was set at a log-rank p-value of less than 0.05.
The UALCAN web tool was utilized to study the expression differences of GBP between normal and tumor tissues at the mRNA level in BrCa. As displayed in Figures 1 and 2, the mRNA expressions of GBP3/5/7 were higher in BrCa tissues compared to normal tissues (GBP3, p = 3.25E-08; GBP5, p = 1.62E-12; GBP7, p = 2.20E-04), whereas the transcription levels of GBP2/4/6 were lower (GBP2, p = 1.62E-12; GBP4, p = 3.316300E-02; GBP6, p = 4.39E-01). These results were confirmed by the TIMER database, which uses TCGA data.
3.2 Relation of GBP mRNA Levels with Clinicopathological Features in BrCa Patients
We further examined the relationship between the mRNA expression of individual GBP members and BrCa clinicopathological features. Higher SBR grades were related with higher mRNA levels of GBP1/2/3/4/5/6 (p < 0.0001). Our study did not indicate a relationship between GBP7 expression and SBR grade in BrCa patients via the bc-GenExMiner v4.8 databases. As shown in Table 1, significant relationships were demonstrated between GBP1/2 expression and the age of the patients (p = 0.02 and p = 0.01, respectively), and nodal status showed a significant relation with GBP1 expression in BrCa patients (p = 0.001). Associated with ER and PR-positive BrCa, the mRNA levels of GBP1/3/4/5/6 were higher in BrCa (p < 0.001). HER-2 showed a significant mRNA level for GBP7 (p = 0.0006). Triple-negative BrCa (TNBrCa) showed significant relationships with GBP1/3/4/5/6/7 mRNA levels (p < 0.0001, p = 0.0005, p = 0.035, p < 0.0001, p = 0.03, p = 0.02, respectively). Basal-like BrCa indicated significant relationships with GBP1/3/4/5/6/7. The mRNA levels of all GBP members showed significant relationships with BrCa PAM50 subtypes (p < 0.0001).
3.3 Immune cell infiltration images of Distinct GBP Family Members in Breast Cancer
We observed the protein expression patterns of GBPs in BrCa using the HPA database. GBP2/3/4/7 and GBP5 showed medium and low staining in breast cancer tissue, respectively. However, GBP1 and GBP6 were not detected in breast cancer tissues. GBP1/2/3/4 and 7 showed medium staining in normal tissue (Figure 3).
3.4 Functional Enrichment of the Top Hundred Related Genes of Distinct GBP
To accurately identify the possible effects of separate GBP in BrCa development, we showed Reactome, GO, and KEGG functional enrichment studies of the top hundred associated genes of distinct GBP by Metascape, which was analyzed by the GEPIA database.
3.5 Relation Between Distinct GBP Expression and Immune Infiltration in BrCa
To assess this theory, we performed an association study of distinct GBP expressions with the abundance of immune cells in BrCa and their molecular subunits by TIMER (Figure 4). Our outcomes indicated that high GBP1/4/5 expression was highly correlated with greater infiltrating abundances of dendritic cells, B cells, CD4+ cells, CD8+ cells, and neutrophils in BrCa. GBP2/3/6/7 expression was weakly positively connected with these immune cell infiltration abundances in BrCa. All GBP expressions and immune infiltration levels were significantly correlated.
3.6 Correlation Between Distinct GBP Expression and Immune Cell Biomarkers in BrCa
To further confirm the relationship between GBP expression and various immune infiltrating cells, we studied the relationship between biomarkers of tumor-infiltrating immune cells and GBP expression in BrCa and its molecular subclasses by TIMER. Our data showed a positive correlation between GBP1/GBP2/GBP3/GBP4/GBP5/GBP6/GBP7 expression and all immune cell infiltration (Figure 5).
3.7 Prognostic Value of GBP Members in BrCa Patients
The prognostic meaning of GBP members in BrCa was studied by the Kaplan-Meier plotter dataset. All GBP members were significant for RFS based on the KMPlotter mRNA gene chip expression of the GBP family in BrCa patients. The anticipated Affymetrix IDs were usable: 202270_at (GBP1), 242907_at (GBP2), 223434_at (GBP3), 1421104_at (GBP4), 238581_at (GBP5), 1559607_at (GBP6), and 1559607_at (GBP7).
We first examined the prognostic values of the seven GBPs in all BrCa patients. As revealed in Figure 1, the mRNA expressions of GBP2/3/4/5/6 were clearly associated with favorable RFS in all BrCa patients, whereas GBP1 expressions were obviously related to unfavorable RFS.
Table 1. The correlation between mRNA level GBPs and clinicopathological feature of BrCa patients (bc-GenExMiner v4.2).
| Criteria | Age | Nodal Status | ER (IHC) | PR (IHC) | HER2 (IHC) | Triple-negative BC (TNBC) | Basal-like BC | ||||||||
| ≤51 | >51 | (-) | (+) | (-) | (+) | (-) | (+) | (-) | (+) | Not | TNBC | Not | Basal-like | ||
| GBP1 | No. | 267 | 476 | 332 | 358 | 530 | 187 | 470 | 243 | 396 | 109 | 578 | 87 | 605 | 136 |
| Mean | 3.6389 | 3.3953 | 3.6412 | 3.3000 | 3.1736 | 4.3559 | 3.2175 | 3.9974 | 3.5805 | 3.6354 | 3.2365 | 4.5715 | 3.1984 | 4.7357 | |
| P-Value | 0.0222 | 0.0018 | <0.0001 | <0.0001 | 0.6812 | <0.0001 | <0.0001 | ||||||||
| GBP2 | No. | 267 | 476 | 322 | 358 | 530 | 187 | 470 | 243 | 369 | 109 | 578 | 87 | 605 | 136 |
| Mean | 4.0006 | 3.8151 | 3.9230 | 3.8316 | 3.8428 | 3.9948 | 3.9025 | 3.8549 | 3.9335 | 3.9909 | 3.8609 | 3.9866 | 3.8820 | 3.8714 | |
| P-Value | 0.0163 | 0.2464 | 0.1140 | 0.5902 | 0.5831 | 0.3541 | 0.9281 | ||||||||
| GBP3 | No. | 267 | 476 | 322 | 358 | 530 | 187 | 470 | 243 | 369 | 109 | 578 | 87 | 605 | 136 |
| Mean | 2.9991 | 3.0528 | 3.0499 | 2.9601 | 3.1305 | 2.7150 | 3.1561 | 2.7676 | 2.9786 | 3.0942 | 3.1241 | 2.5798 | 3.1117 | 2.6861 | |
| P-Value | 0.5845 | 0.3627 | 0.0003 | 0.0002 | 0.4092 | 0.0005 | 0.0010 | ||||||||
| GBP4 | No. | 267 | 476 | 322 | 358 | 530 | 187 | 470 | 243 | 369 | 109 | 578 | 87 | 605 | 136 |
| Mean | 2.5961 | 2.6079 | 2.6672 | 2.5087 | 2.4994 | 2.8714 | 2.5410 | 2.7064 | 2.6865 | 2.6922 | 2.5292 | 2.9367 | 2.5161 | 2.9879 | |
| P-Value | 0.9147 | 0.1535 | 0.0081 | 0.1792 | 0.9684 | 0.0352 | 0.0083 | ||||||||
| GBP5 | No. | 267 | 476 | 322 | 358 | 530 | 187 | 470 | 243 | 369 | 109 | 578 | 87 | 605 | 136 |
| Mean | 0.8667 | 0.7929 | 0.9305 | 0.6635 | 0.4962 | 1.7325 | 0.5381 | 1.3611 | 0.9000 | 1.0523 | 0.5673 | 1.9679 | 0.5453 | 2.0170 | |
| P-Value | 0.5928 | 0.0535 | <0.0001 | <0.0001 | 0.3897 | <0.0001 | <0.0001 | ||||||||
| GBP6 | No. | 267 | 476 | 322 | 358 | 530 | 187 | 470 | 243 | 369 | 109 | 578 | 87 | 605 | 136 |
| Mean | -1.8426 | -1.9458 | -1.9058 | -1.9212 | -2.0054 | -1.6363 | -2.0040 | -1.7217 | -1.8606 | -1.8539 | -1.9838 | -1.6616 | -2.0038 | -1.5015 | |
| P-Value | 0.2176 | 0.8595 | 0.0006 | 0.0046 | 0.9546 | 0.0383 | 0.0002 | ||||||||
| GBP7 | No. | 267 | 476 | 322 | 358 | 530 | 187 | 470 | 243 | 369 | 109 | 578 | 87 | 605 | 136 |
| Mean | -2.6550 | -2.6349 | -2.6030 | -2.6823 | -2.6721 | -2.5890 | -2.6564 | -2.6343 | -2.6043 | -2.7909 | -2.6775 | -2.5058 | -2.6662 | -2.5318 | |
| P-Value | 0.5652 | 0.0712 | 0.1195 | 0.6348 | 0.0006 | 0.0218 | 0.0435 | ||||||||
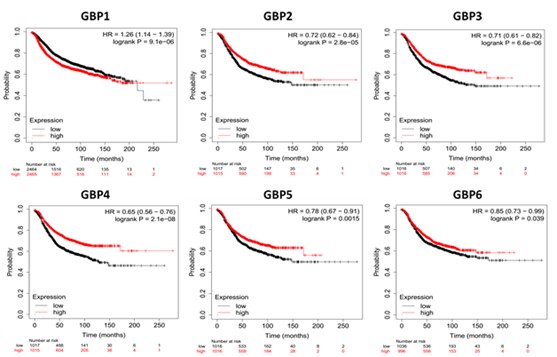
Figure 1. Kaplan-Meier Curve of relapse free survival based on KMPlotter mRNA gene chip expression of GBP Family in BrCa patients. Red line represents higher expression and black line represents lower expression.
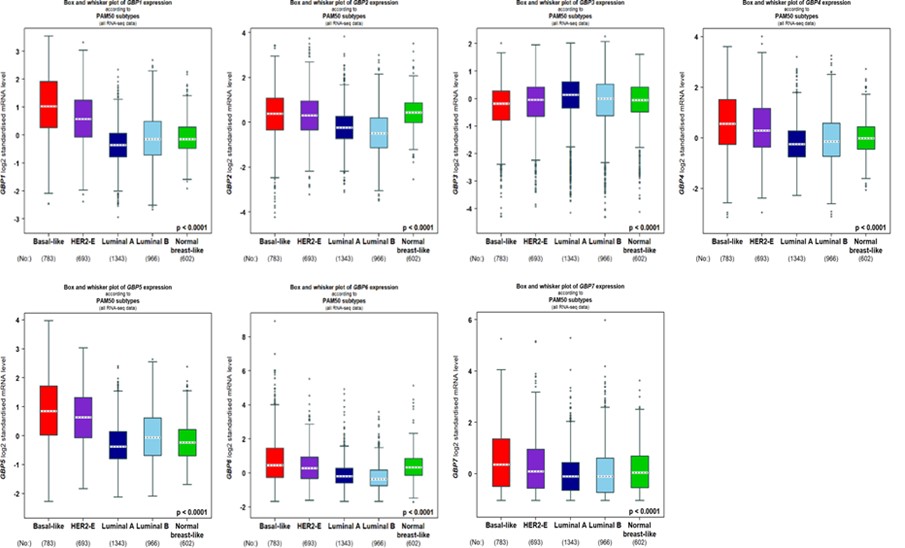
Figure 2. Molecular expression analysis of GBP Family in breast cancer generated from bc-GenExMiner v4.8. Molecular subtyping of breast cancer patients according to PAM50 subtypes HER2-E human epidermal growth factor receptor, PAM50 Parker’s molecular.
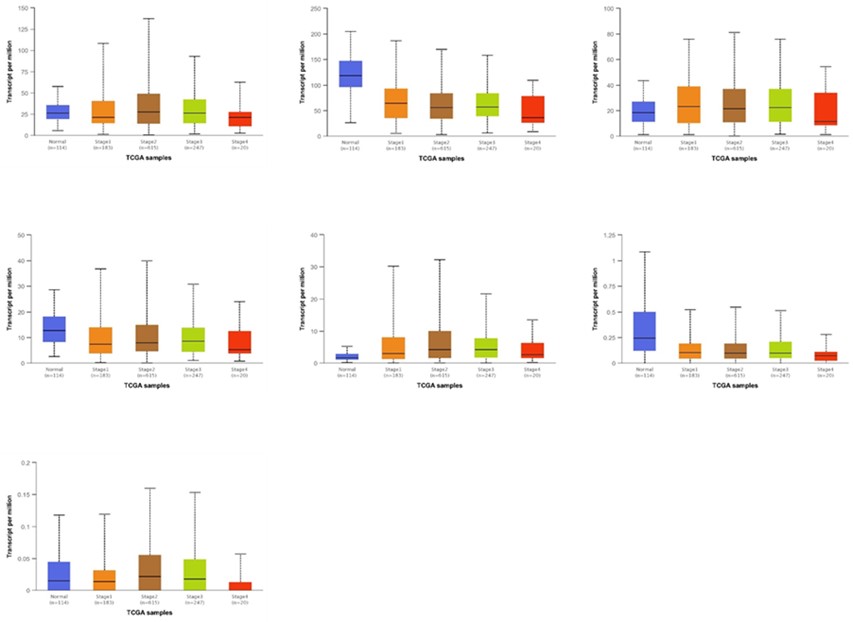
Figure 3. GBP Family profile based on different variables of TCGA breast cancer retrieved from UALCAN web. A Sample types (***p < 0.03), B individual cancer stages.
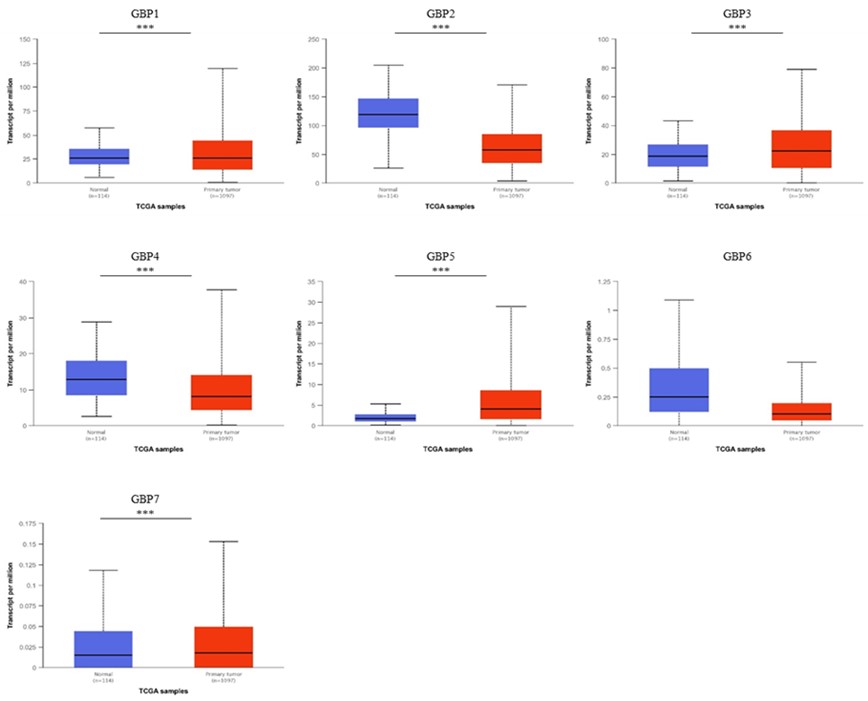
Figure 4. GBP Family profile based on different variables of TCGA breast cancer retrieved from UALCAN web. A Sample types (***p < 0.03), B individual cancer stages.
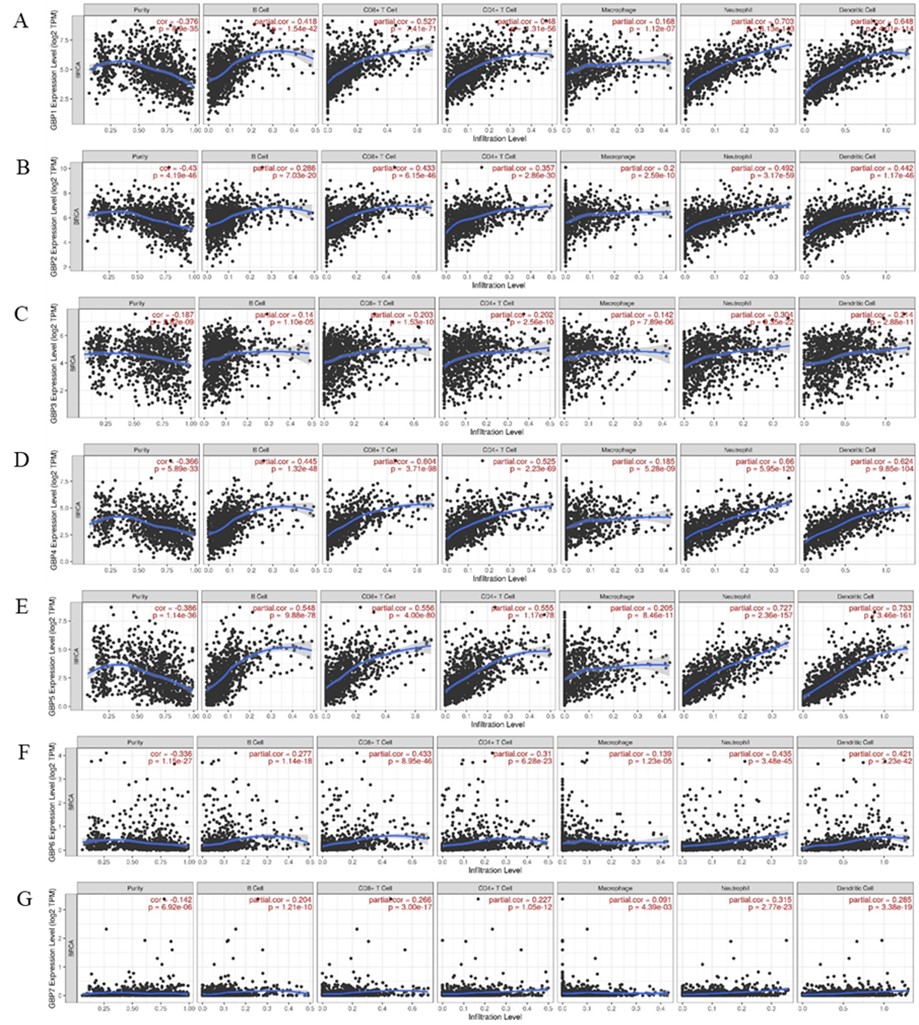
Figure 5. The correlation between different expressed GBP Family and immune cell infiltration (TIMER). The correlation between the abundance of immune cell and the expression of (A) GBP1, (B) GBP2, (C) GBP3, (D) GBP4, (E) GBP5, (F) GBP6, and (G) GBP7 in BC.
Discussion
BrCa is one of the most widespread malignancies among women worldwide, with more than 570,000 mortalities reported in 2015. Each year, around 1.5 million women worldwide (25% of entirely malignance cases) are diagnosed with BrCa (24). Several risk factors for breast cancer including sex, estrogen, family history, aging, gene mutations, and leading an unhealthy lifestyle (24, 25).
Interferon-inducible guanylate-binding protein (GBP), which is regulated by p53 and suppresses Rac protein and NF-Kappa B, as well as the expression of matrix metalloproteinase 9, has been recently mentioned as a probable control factor in tumor formation (26). GBP is a significant dynamin superfamily GTPase involved in regulating cytoskeleton, membrane, and cell cycle dynamics. In various cell types, including monocytes and endothelial cells, GBP1 expression is triggered by IFNγ and functions to restrain cellular proliferation in inflammatory situations. GBP1 is vital for the autophagosomes’ maturation in response to pathogens and intracellular pathogen-associated molecular designs in immunity. GBP1 also prevents endothelial cell proliferation while protecting against IFNγ-induced apoptosis in chronic inflammation (27). Similar proliferation inhibition has been observed in several tumor models, including breast, colorectal, and prostate carcinoma mouse models. However, the current research is the initial to thoroughly discover the potential function and prognostic rate of GBP in BrCa.
GBP1 is noticeable among the complex proteins, and its expression seems to be upregulated in primary tumor examples and brain metastasis. Silencing GBP1 meaningfully decreased the skill of BrCa cells to cross the blood-brain barrier (28). These studies displayed that unfavorable RFS were clearly correlated with GBP1 expressions. Additionally, GBP1 expression is strongly associated with dendritic cells, CD8+ cells, B cells, CD4+ cells, and neutrophil infiltration abundances in BrCa (29). Quintero et al. demonstrated that knocking down GBP1 significantly impacts the development of triple-negative BrCa (TNBC) cell lines. Furthermore, we indicated that in breast cancer cell lines, EGFR was responsible for regulating GBP1 expression (29). Additionally, ovarian cancer patients' progression-free survival is shortened by elevated GBP1 levels. Overall, the findings revealed that GBP1 directly suppresses tumor growth in CRC and that the reduction of GBP1 expression may indicate the tumor is avoiding the Th1 immune response dominated by IFNγ (30).
GBP2 is a member of the p65 GBP family of interferon-gamma-inducible GTPases that regulate the innate immune reaction to microbial infections (16). GBPs were first discovered in macrophages and are the most plentiful proteins in cells visible to interferon. GBP2 influences other cell processes, such as cell spreading and proliferation, in addition to its role in the innate immune reaction (16). Expressing mouse GBP2 in NIH3T3 fibroblasts reduces cell doubling time. GBP2 was recently identified as a TGF-β target gene activated in metastatic breast cancer cells (17). Our study showed that a higher SBR level was related to GBP2 mRNA levels. GBP2 also showed a weak association with immune infiltrating cells (19). Wang et al. found that guanylate-binding protein-2 prevents colorectal cancer cell growth and rises sensitivity to paclitaxel in resistant colorectal cancer cells by interfering with Wnt signaling. GBP2 expression also prevented Rac activation and cell spreading (31). It suppresses Akt activation by forming a protein complex with p110, the catalytic subunit of phosphatidylinositol 3-kinase (31). STAT1 and IRF1 transcription factors are essential for GBP2 transcriptional activation. GBP2 might have a role in tumor growth by preventing NF-Kappa B and Rac protein, thereby blocking the induction of matrix metalloproteinase-9 (MMP-9) (20). GBP2 expression was also shown to be developed in esophageal squamous cell carcinoma rather than in surrounding normal epithelium. By interacting with IRF1, p53 upregulates GBP2 expression (21).
GBP5 is a member of the interferon-gamma (IFN-γ)-inducible large GTPase family elaborate in various cellular processes, comprising inflammasome stimulation and innate immunity in contradiction of multiple microbial pathogens (32, 33). GBP5 has been linked to favorable prognosis and immune infiltrations in PD-1 and PD-L1 over-expressing basal-like breast cancers (34). Our outcomes showed that GBP5 was related to favorable RFS in all breast cancer patients. GBP5 also showed low staining in BrCa tissue. Hunt et al. indicated that in HER2+ breast tumors, GBP5 was associated with better RFS (35).
Conclusion
In conclusion, our findings may contribute to developing new therapeutic treatments, helping clinicians select appropriate medications for their patients with breast cancer, and identifying prognostic biomarkers that more precisely predict the BrCa patient's survival.
Declarations
Acknowledgements
Not applicable.
Ethical Considerations
Not applicable.
Authors' Contributions
All authors participated in gathering the data and review of the manuscript. All authors have reviewed the manuscript and given their approval for publication.
Conflicts of Interest
The authors declare no conflict of interest.
Fund or Financial Support
This research received no specific grant from any funding agency in the public, commercial, or not for profit sector.
Using Artificial Intelligence Tools (AI Tools)
The authors were not utilized AI Tools.
Received: 1901/12/14 | Accepted: 2025/02/27 | Published: 2025/03/13
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |







