BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://journal.zums.ac.ir/article-1-7355-en.html
2- Dept. of Chemistry, College of Science, University of Thi-Qar, Thi-Qar, Iraq
3- Technical Institute Al-Nassiriah, Southern Technical University, Thi-Qar, Iraq
✅ The results of this study point out that the S. platensis phenolic extract has anticancer potential, and the phytoconstituents contributed to the anticancer effects.
Algae is one of the richest biological sources used to treat microbial, inflammatory, fungal, cancer, viral infections, rheumatism, and AIDS. They have biologically effective compounds that are derived from secondary metabolism. Also, the source of many of them, such as fatty acids, carotenoids, phenols, chlorophyll, alkaloids, terpenes, and other compounds, are used in research, manufacturing, and development of medicines (1). Nowadays, one of the main causes of death in the world is cancer. Cancer is a preventable disorder but has evolved with a significant response to modulation via nutritional factors. Cancer can occur in any body tissue and has many forms in each body area (2).
On the other hand, due to the side effects of cancer treatment and the acquired drug resistance, there is a great need for new methods and strategies and effective anticancer agents to improve the patients and the success of cancer treatment, and one of those methods is the combination of existing anticancer drugs with polyphenols. Researchers have found that natural phenolic compounds have an essential effect in treating and preventing cancer. Many studies have been conducted in this regard, showing that dietary phenols' beneficial effects involve anti-clastogenic, anti-inflammatory, and antioxidant activities (3).
A lot of research has been tested in vitro conditions and in vivo to prevent cancer progression and growth, which has been studied about the ability of rich crude extracts of fractions containing mixtures or phenolic compounds (4-6).
The anti-carcinogenic effects of phenolic compounds regulate pro- and anti-inflammatory cascades and ROS levels, assist proper cell distinctness and correct development and advance the transcription of tumor suppressor proteins such as p53. Also, the anticancer effects of phenolic compounds are primarily because of the capability and potentiality to induce cell cycle arrest and prevent oncogenic signaling cascades, cell proliferation, angiogenesis, and apoptosis excitation (7).
According to the findings of Abdelhakim et al., phenolic compounds provide a wide range of prophylactic and curative options against various types of cancer. These compounds can be utilized singly or in combination with other anticancer drugs. Some phenolic compounds, such as gallic acid and quercetin, are well-known and demonstrate performance mechanisms. These molecules mainly take action on the various checkpoints of cancer cells. They are a great resource of natural anticancer substances (8).
The cytotoxicity results of Mansur et al. revealed polyphenols extracted from the brown alga Cystoseira tamariscifolia (now identified as Carpodesmia tamariscifolia) reduce cell viability and inhibit cell growth in human leukemia, THP-1, and HL60 and prostate cancer PC3 cell lines in a concentration-dependent manner (9).
Studies have been conducted on different types of cancer, some of which demonstrate that phenolics are very effective candidates in treating various types of cancer, so they act on other molecular targets such as proliferation, angiogenesis, growth and differentiation, metastasis, and apoptotic (10). The present study is focused on evaluating the anticancer influence of the phenolic extract of Spirulina platensis against human esophagus cancer cell SK-GT-4 and HBL-100 normal human breast epithelial cells to establish anticancer activity.
Source of Spirulina platensis
S. platensis was purchased in powder form from ALIBAB Company, and before use, it was stored in airtight bags at ambient temperature in the dark.
Extraction of Phenolic Compounds from Spirulina platensis
Extraction was carried out based on the principle and procedure developed by Altemimi et al. (11) and Kredy et al. (12) with some modifications. Each 40 g of S. platensis was placed in brown bottles with 500 mL ethanol (70%) added. The mixture was put in an ultrasonic bath, and then the mixture was filtered in a Buchner funnel through Whatman No. 1 filter paper utilizing a vacuum pump. Then, the filtrate solution was evaporated through a rotary evaporator under reduced pressure at 40°C and then dried. After that, the alcoholic extract was dissolved in 30 mL of distilled water, and then 50 mL of chloroform was added to it in a separation funnel to obtain two layers (water and organic layer); the step was repeated by adding 50 mL of chloroform to the aqueous layer, stirring for 48 hours on a magnetic stirrer. Then, the mixture was transferred to a separation funnel; the aqueous layer was separated. After that, the aqueous layer obtained from the fourth step was extracted with the same volume of ethyl acetate with stirring for 48 h on a magnetic stirrer; after that, the mixture was transferred to a separation funnel to obtain two layers (aqueous and ethyl acetate layer), the ethyl acetate layer was separated and dried to obtain the phenolic extract.
Qualitative Tests for Preliminary Phytochemical Screening of Phenolic Extract from Spirulina platensis
Preliminary phytochemical screening for phenols, alkaloids, glycosides, flavonoids, triterpenoids, carbohydrates, triterpenes, saponins, tannins, triterpenes, and sterols was carried out for Phenolic extract from S. platensis following standard methods previously reported for their identification and confirmation (13-15).
Determination of Phenolic extract by HPLC
Phenols isolated from S. platensis were identified using the HPLC (High-Performance Liquid Chromatography) column in laboratories of Technology and the Ministry of Science. Reversed-phase HPLC processing was utilized to quantify singular phenolic compounds using a SYKAMN HPLC chromatographic device fitted with a UV detector, Chemstation, and a Zorbax Eclipse Plus-C18- OSD. 4.6 mm column, 25 cm, the temperature in the column was 30°C. With eluents A (methanol) and B (1% formic acid in water (v/v)), the gradient elution procedure was carried out as follows: 40% B for the first 0–4 min; 50% B for the next 4–10 min; and a flow rate of 0.7 mL/min. An autosampler automatically injected 100 μL of samples and 100 μL of standards. The spectra were taken at a wavelength of 280 nm (16).
Cell Lines
The cell lines, human esophageal cancer cell SK-GT-4, and normal human breast epithelial cell HBL-100, were acquired from the tissue culture lab at Basra University's College of Education for Pure Sciences.
Cell Culture
The cells were cultured in an incubator with a humidified 5% CO2 atmosphere at 37°C using RPMI-1640 media with 10% FBS, 100 V/ mL of penicillin, and 100 μg/ mL of streptomycin. After 90% confluency, cells were treated with 0.25% sterile trypsin and seeded into 96-well microplates at a density of 1.0×104 cells/well for 24 hours before including test substances (17).
Cytotoxicity
The cytotoxicity values of extracts were determined by the MTT assay (18). In this assay, cancer and normal cells were seeded in 96-well plates at a density of 1×104 cells per well and incubated for 24 h at 37°C with 5% CO2. The cells were then treated with different concentrations (15.5, 31, 62.5, and 125 μg/mL) of phenolic extract. Four replicates were used for each concentration. Four wells were used for seeding cells in a maintenance medium with PBS as a control. The plates were re-incubated at 37°C, 5% CO2 incubator for 72hr. After exposure, the extracts and media were removed from the plates and washed with PBS at 37°C. Then, 100 µL of MTT solution (10 µL of MTT and 90 µL of media-free serum) was added to each well, and cells were incubated for 2 hours at 37°C. The MTT solution was discarded, and 100 µL of Dimethyl sulfoxide DMSO was added to each well and incubated for 20 minutes in the dark to dissolve insoluble formazan crystals. Cell viability was measured in terms of absorbance at 620 nm by ELISA plate reader, and the decrease in cell viability was expressed as the percentage compared with the control group.
The cell viability was calculated by the following formula:
The Cell Viability (%) = (Abs T/ Abs C) ×100
Where Abs C is the Absorbance of the Control, and Abs T is the absorbance of the Test sample
The IC50 for each extract was counted using a graph pad prism program (19).
Statistical Analysis
The findings are shown as a mean and standard error of means, One-way ANOVA was used to find changes between the cell lines of the treatment and control groups. Statistics were deemed to be significant for values with a P-value< 0.05.
Chemical Reagent of the Extract
Generally, the phenolic extract contains phenolic acids, flavonoids, carbohydrates, tannins, and glycosides. In contrast, it did not include sterols, terpenoids, and terpenes. The results also showed that the extract does not contain alkaloids and saponins. The results of qualitative tests are shown in Table 1.
Table 1. Qualitative tests of some active compounds in the phenolic extract of Spirulina platensis
| Active principle | Test | Results |
| Phenolic Compounds | FeCl3 (1%) + ammonia vapor | + |
| Flavonoids | Alcohol KOH | + |
| Carbohydrates | Molisch | + |
| Alkaloids | Wagner | - |
| Tannins | Pb(AC)3 (1%) | + |
| FeCl3 (1%) | + | |
| saponins | HgCl2 (1%) | - |
| Triterpenoids | CH3Cl+H2SO4 | - |
| Glycosides | Before analysis | + |
| After analysis | + | |
| Triterpins and Setrols | Liebermann – Burchard | - |
HPLC Analysis
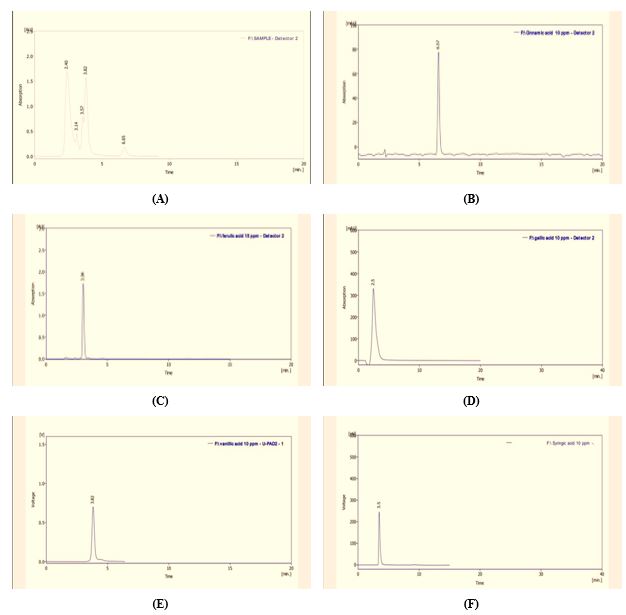
The extract was analyzed by HPLC to estimate the contents of phenolic compounds. Figure 1(a-f) explains that the retention time of the sample agrees with the standard retention time of most contents in the extract.
Cytotoxicity of the phenolic extract on cell lines:
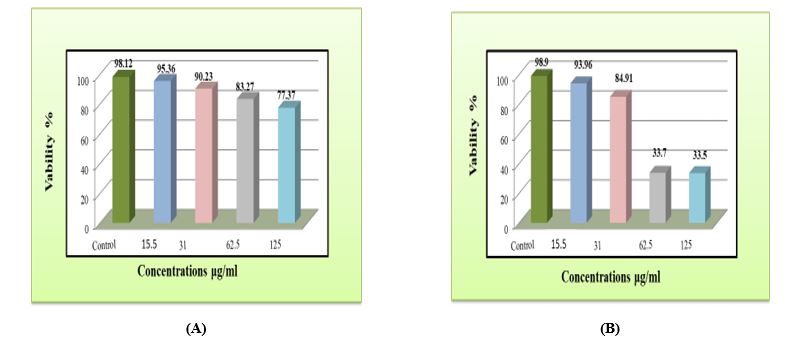
The results of the cytotoxicity assay in the current study revealed the effect of the phenolic extract of S. platensis on the proliferation of HBL100 and SK-GT-4 cell lines which has been shown in Figure 2(a-b).
Table (2) shows no significant difference in the viability percentage of cancer cell line SK-GT-4 between the control group and concentration (15.5 μg/mL) (P<0.05). There was a significant decrease in the viability percentage of cancer cell line SK-GT-4 at a concentration (31.0 μg/mL) in comparison with the control group and concentration (15.5 μg/mL) (P<0.05).
Also, there was a significant decrease in the viability percentage of cancer cell line SK-GT-4 at concentrations (62.5 and 125.0 μg/mL) in comparison with the control group and concentrations (15.5 and 31.0 μg/mL) (P<0.05). The same table shows no significant difference in the viability percentage of cancer cell line SK-GT-4 between concentrations (62.5 and 125.0 μg/mL) (P<0.05). The IC50 value for cancer cell line SK-GT-4 is 36.52 µg/mL.
Figure 1. HPLC of (a) alcoholic extract from S. platensis, (b) standard Cinnamic acid, (c) standard Ferulic acid, (d) standard gallic acid, (e) standard Vanillic acid, (f) standard Syringic acid
Table 2. The significant differences in viability percentage of cancer cell line SK-GT-4 after being treated with phenolic extract of S. platensis
| SK-GT-4 | Concentration µg/mL | ||||
| Control | 15.5 | 31.0 | 62.5 | 125.0 | |
| Mean ±SD | 98.90 ± 0.24a | 93.96 ± 7.21a | 84.91 ± 9.47b | 33.70 ± 1.29c | 33.50 ± 1.56c |
| LSD | 6.69 | ||||
| P-value | 0.001 | ||||
Note: Each value represents mean SD values with non-identical superscript (a, b or c) were considered significant difference P<0.05 SD: Standard Deviation, LSD: Less Significant Difference.
Table 3 shows no significant difference in the viability percentage of normal cell line HBL100 between the control group and concentrations of 15.5, 31.0, and 62.5 μg/mL, (P<0.05). There was a significant decrease in the viability percentage of normal cell line HBL100 at concentrations 125.0 μg/mL in comparison with the control group and concentrations 15.5 µg/mL (P<0.05). Table 3 also shows no significant difference in the viability percentage of normal cell HBL100 between concentrations of 31.0, 62.5, and 125.0 μg/mL, (P<0.05). The IC50 value for cancer cell line SK-GT-4 is 36.52 µg/mL.
Table 3. The significant differences in viability percentage of normal cell line HBL100 after being treated with phenolic extract of S. platensis
| HBL100 | Concentration µg/mL | ||||
| Control | 15.5 | 31.0 | 62.5 | 125.0 | |
| Mean ±SD | 98.12± 0.90 a | 95.36 ± 22.9 a | 90.23 ± 6.99 ab | 83.27 ± 8.54 ab | 77.37 ± 11.85 b |
| LSD | 15.59 | ||||
| P-value | 0.171 | ||||
Note: Each value represents mean SD values with non-identical superscript (a,b or c )were considered significant difference P<0.05 SD: Standard Deviation, LSD: Less Significant Difference
Figure 2. The Effect of Different Concentrations of Phenolic Extract of S. platensis on (a) Normal Human Breast Epithelial Cell Line HBL100, (b) Esophagus Cancer Cell Line SK-GT-4 after Incubation for 72 Hours
Discussion
Phenolic compounds derived from algae are of major interest in the continuous search for more assured, safer, and more effective therapies than the use of drugs. Also, phenolic compounds have the meaningful ability and potential as cytotoxic anticancer drugs because they target various cancer components, enhancing apoptosis and reducing its proliferation (angiogenesis, growth, differentiation, and metastasis) (20). Our results showed that phenolic extract contains several compounds because of the use of ethanol/water as a high-selectivity solvent of phenolic compounds. The extraction of phenolic compounds relies on the polarity of the solvents; an individual solvent may not be efficient for deriving a bioactive compound. Therefore, combining alcohol with water is more efficient in the extraction of phenolic compounds than alcohol singly (21). The findings of the current research are in complete agreement with the previous researches. According to the reports, adding water to alcohol demonstrates a synergistic effect and enhances the functioning of the extraction of phenolic compounds from the samples (22). Many phenolic compounds are ordinarily present as glycosides in plants (23). HPLC results demonstrated that the phenolic extract of S. platensis includes some significant compounds that contain ferulic acid, gallic acid, cinnamic acid, syringic acid, and vanillic acid. Some unknown compounds are demonstrated on the peaks of the mentioned chromatogram, which are likely to be derivatives of phenolic compounds. The liquid chromatography method has been widely approved and accepted by many researchers, and it is a very efficient and useful tool for extracting and analyzing phenolic compounds. Liquid chromatography is a method of separation and determination of characteristics with high performance and efficiency. In this method, HPLC is combined with various detectors for the analysis of phenolic compounds, such as a photodiode array detector (PDA) and ultraviolet-visible (UV) (24). Each compound was identified based on retention time compared to pure commercial standards (25).
The MTT assay results indicated that the anticancer activity of the phenolic extract increased with increasing concentration of the extract. Therefore, our results suggest that the Phenolic extract of S. platensis was toxic to cancer cell line SK-GT-4, whereas, in normal cell line HBL100, the toxicity was too low. These results agree with Akbarizare et al.'s studies, which concluded that phenolic compounds extracted from S. platensis show anticancer activity against liver cancer cell line HepG2. At the same time, normal human fibroblasts are unaffected (26).
The results of HPLC shown in Figure 1(a) for a phenolic extract of S. platensis indicate the presence of effective phenolic compounds in the phenolic extract, such as vanillic acid, ferulic acid, and gallic acid, which have an inhibitory role in cancer cells. This is consistent with Bortolini et al. and Kumar et al. (27, 28) studies.
Many findings have demonstrated the anticancer properties of these compounds because these compounds have the potential and ability to prevent the growth of tumor cells and induce apoptosis and anti-proliferation (29-31). Moreover, various biological interactions and processes occur between action phenolic compounds or polyphenols, flavonoids, and enzymes and proteins when they make them toxic to cells or prevent cell growth. The studies show that the anticancer properties of natural plants and cytotoxicity play an important role in preventing chemotherapy by showing the effects of cell angiogenesis and signal transmission, which is due to the existence of flavonoid composition (32). According to Wahla et al., phenolic compounds should be studied and researched in various fields, including inhibition of resistance to these therapies, radiotherapy, and increasing the efficiency of conventional chemotherapy treatment regimens. Phenolic compounds can limit angiogenesis the formation of new blood vessels needed for tumor growth. Also, these compounds can promote and improve the potential and capacity of the body's immune system to recognize and destroy cancer cells. In addition, they decrease cancer cell invasion and adhesion, which reduces their potential for metastasis (33). The findings reveal that the hydroxyl radical is the most efficient and effective free radical among all ROS. These studies suggest that it can cause significant biological damage and lipid oxidation, which may lead to cancer (20). It is predicted that more studies will be conducted on other cancer cell lines to comprehend the full curative potential of the active substances available in this type of algae and molecular apoptosis of cancer cells and biochemical mechanisms.
Conclusion
According to the MTT assay, phenolic extract from S. platensis inhibited the growth of esophageal cancer cells SK-GT-4 and normal human breast epithelial cells HBL100. SK-GT-4 cells were toxic to the phenolic extract of S. platensis, with an IC50 value of 36.52 µg/mL. In contrast, the toxicity was low in regular cell line HBL100. Also, data analysis showed that phenolic extracts from S. platensis could be further developed as natural anticancer agents effective against esophagus carcinoma. However, more clinical trials and confirmatory studies need to be conducted before they can be used to treat esophagus carcinoma.
Acknowledgements
None.
Funding
This research received no external funding.
Ethics and RCT Code
This study was conducted following the ethical principles outlined by the Ministry of Health, Iraq, and received ethical approval from the Ethics Committee/ College of Science.
Authors' contributions
Conceptualization, MA and HA; methodology, MA; software, RA; validation, MA, HA, and RA; writing-original draft preparation, MA, HA, and RA; writing-review and editing, MA, HA, and RA; visualization, HA; supervision, MA and HA; project administration, RA".
Conflicts of Interest
The authors declared no conflict of interest.
Received: 2023/08/8 | Accepted: 2023/10/20 | Published: 2023/10/29
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |